The iodide ion reacts with hypochlorite ion (the active ingredient in chlorine bleaches) in the following way:
OCl−+I−→OI−+Cl−.
This rapid reaction gives the following rate data:
[OCl−](M) [I]−(M) Rate (M/s)
1.5×10^−3 1.5×10^−3 1.36×10^−4
3.0×10^−3 1.5×10^−3 2.72×10^−4
1.5×10^−3 3.0×10^−3 2.72×10^−4
a. Write the rate law for this reaction.
b. Calculate the rate constant with proper units.
c. Calculate the rate when [OCl-]= 1.8×10^3 M and [I-]= 6.0×10^4 M .
Solved
Show answers
More tips
- F Family and Home When and how to start introducing solid foods to your baby?...
- B Business and Finance Moneybookers – What it is and How it Works...
- C Computers and Internet How to Format Your C Drive: Detailed Guide and Tips...
- F Food and Cooking What can and cannot be eaten during Lent?...
- H Health and Medicine What to Do When Your Jaw Locks Up?...
- F Family and Home Why Having Pets at Home is Good for Your Health...
- D Dating, Love, Relationships Is it a Compliment or Flattery: What s the Difference?...
- S Science and Technology The Metric System in Our Daily Life: Understanding Its Importance...
- C Computers and Internet What to Do If Your ICQ Gets Hacked?...
- C Computers and Internet How to Choose a Monitor?...
Answers on questions: Chemistry
- G Geography Starting from the middle of the Earth to the outside of the Earth, what are the theoretical internal structures of the Earth...
- M Mathematics Will Mark Branliest What is the approximate mean absolute value for the data shown in the dot plot? 1.09 0.92 2 6...
- E English How is a tsunami different from an earthquake?...
- M Mathematics The following input-output pairs have been observed during the operation of a linear system: x1(n) = f−1; 2; 1g ! t y1(n) = f1; 2; −1; 0; 1g x2(n) = f1; −1; −1g ! t y2(n) =...
- M Mathematics the high school basketball team has 14 players: 2/7 are guards, 1/2 are forwards, and the rest are centers. find the number of players in each position on the team. show your...

Ответ:
a) The rate law for this reaction:
b)The rate constant of the reaction is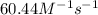 .
.
c)The rate of the reaction at given concentration will be .
.
Explanation:
a) Rate law of the reaction is given by :
1) Rate of the reaction when![[OCl^-]=1.5\times 10^{-3} M](/tpl/images/0518/3250/b77da.png) and
and ![[I^-]=1.5\times 10^{-3} M](/tpl/images/0518/3250/2f2e0.png) .
.
2) Rate of the reaction when![[OCl^-]=3.0\times 10^{-3} M](/tpl/images/0518/3250/3317d.png) and
and ![[I^-]=1.5\times 10^{-3} M](/tpl/images/0518/3250/2f2e0.png) .
.
3) Rate of the reaction when![[OCl^-]=1.5\times 10^{-3} M](/tpl/images/0518/3250/b77da.png) and
and ![[I^-]=3.0\times 10^{-3} M](/tpl/images/0518/3250/b24ad.png) .
.
[1] ÷ [2]
On solving the equation we get value of x :
x = 1
[1] ÷ [3]
On solving the equation we get value of y :
y = 1
The rate law for this reaction:
b)
Rate of the reaction when![[OCl^-]=1.5\times 10^{-3} M](/tpl/images/0518/3250/b77da.png) and
and ![[I^-]=1.5\times 10^{-3} M](/tpl/images/0518/3250/2f2e0.png) .
.
The rate constant of the reaction is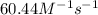 .
.
c)
Let the rate of the reaction when![[OCl^-]=1.8\times 10^{3} M](/tpl/images/0518/3250/b9482.png) and
and ![[I^-]=6.0\times 10^{4} M](/tpl/images/0518/3250/ceced.png) be R.
be R.
The rate of the reaction at given concentration will be .
.
Ответ:
20pcs I hope it help or maybe 30