videogamer1192
13.12.2019 •
Chemistry
The ksp of copper(ii) ferrocyanide (cu2[fe(cn)6]) is 1.3 × 10−16 at 25°c. determine the potential of a concentration cell in which one half-cell consists of a copper electrode in 1.00 m copper(ii) nitrate, and the other consists of a copper electrode in a saturated solution of cu2[fe(cn)6].
ferrocyanide, ([fe(cn)6]4−), is a complex ion.
Solved
Show answers
More tips
- F Family and Home When and how to start introducing solid foods to your baby?...
- B Business and Finance Moneybookers – What it is and How it Works...
- C Computers and Internet How to Format Your C Drive: Detailed Guide and Tips...
- F Food and Cooking What can and cannot be eaten during Lent?...
- H Health and Medicine What to Do When Your Jaw Locks Up?...
- F Family and Home Why Having Pets at Home is Good for Your Health...
- D Dating, Love, Relationships Is it a Compliment or Flattery: What s the Difference?...
- S Science and Technology The Metric System in Our Daily Life: Understanding Its Importance...
- C Computers and Internet What to Do If Your ICQ Gets Hacked?...
- C Computers and Internet How to Choose a Monitor?...
Answers on questions: Chemistry
- C Chemistry Regions at higher elevations, in general, have climates than regions at lower elevations. A. drier B. hotter C. colder D. calmer...
- C Chemistry A sheet of copper is 1 mm thick and has surface dimensions of 5 cm × 29 cm . If the long edges are joined to form a tube 29 cm in length, what is the resistance between the ends?...
- C Chemistry Is a methyl group or any other alkyl group (R) considered to be an ortho & para director or meta director ? Explain your answer by: a. drawing all resonance structures for...
- C Chemistry You flip three coins (a nickel, a dime, and a quarter), assigning the values of +1 for heads (H) and –1 for tails (T). Each outcome after flipping the three coins constitutes a...
- C Chemistry A vision for the public reading answers...
- C Chemistry What is the density of a salt solution if 75.0 mL of the solution has a mass of 32.0 g?...
- C Chemistry I NEEDD SOMEBODY HELP...
- C Chemistry The first person to make me blush gets my email and brainliest NOTE: I saw someone do this if not interested pls don t reply Age: Any Gender: Male Pics Allowed...
- C Chemistry Food cloth rope lumber paper and rubber come from plants true or false due in 10 minutes helppp...
- C Chemistry What limits the growth of phytoplankton? A. the depth of the ocean B. the amount of nutrients in the water C. the amount of water that runs off into the ocean D. the number of...

Ответ:
Explanation:
Expression for of the given reaction is as follows.
of the given reaction is as follows.
Let us assume that the concentration of given species is "s". As the value of is given as
is given as  .
.
s =
Therefore, concentration of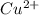 will be calculated as follows.
will be calculated as follows.
=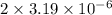
= M
M
Now, we will calculate the value of as follows.
as follows.
=
= 0.1535 V
Thus, we can conclude that the potential of given cell is 0.1535 V.
Ответ: