ryanbransky
09.10.2019 •
Chemistry
The reaction of silver metal and dilute nitric acid proceeds according to the equation above. if 0.10 mole of powdered silver is added to 10.0 milliliters of 6.0 molar nitric acid, the number of moles of no gas that can be formed is
a) 0.015 mole
b) 0.020 mole
c) 0.030 mole
d) 0.045 mole
e) 0.090 mole
Solved
Show answers
More tips
- S Science and Technology Colliders: How They Work and Why They Matter...
- C Construction and repair How to Choose the Best Underfloor Heating?...
- C Computers and Internet How to Get Rid of Windows Genuine Check?...
- C Computers and Internet War of Social Media: Which Platform is the Leader?...
- H Health and Medicine How to Treat the Flu: A Comprehensive Guide...
- O Other What is a Disk Emulsifier and How Does it Work?...
- F Family and Home What does a newborn need?...
- F Family and Home Choosing the Right Car Seat for Your Child: Tips and Recommendations...
- F Food and Cooking How to Get Reconfirmation of Registration?...
- C Computers and Internet How to Get Rid of Spam in ICQ?...
Answers on questions: Chemistry
- M Mathematics Please please help mee!! it’s probability no TROLL COMMENTS PLS OR I WILL REMOVE YOUR ACCOUNT :)...
- M Mathematics 2. which is a disadvantage of purchasing and owning a home? a.an individual assumes all responsibility for maintenance and repair of owned property. b.an individual has more freedom...
- C Chemistry The reaction 2K(s) + Cl2(1) - 2KCl(s) is a(n)reaction.*...
- M Mathematics Explanation pleasee!...
- S Social Studies What does respect mean to you? (5 sentences)...

Ответ:
The moles of NO formed from the given reaction has been 0.015 mol. Thus, option A is correct.
The balanced chemical equation for the reaction has been:
The moles of nitric acid can be given as:
Moles = Molarity × Volume
The molarity of nitric acid = 6 M
Volume of nitric acid = 10 ml = 0.01 L
Moles of nitric acid = 6 × 0.01 mol
Moles of nitric acid = 0.06 mol.
From the balanced equation,
3 moles silver requires = 4 moles nitric acid
0.10 moles silver requires =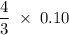 moles nitric acid
moles nitric acid
0.10 moles silver requires = 0.13 mol nitric acid.
Since the available nitric acid is 0.06 mol, it has been in lesser amount and has been the limiting reagent.
Thus,
4 moles nitric acid = 1 moles NO
0.06 mol nitric acid = 0.015 moles NO.
Thus, the moles of NO formed from the given reaction has been 0.015 mol. Thus, option A is correct.
For more information about the moles in a equation, refer to the link:
link
Ответ:
The answer to your question is: 0.015 mole of NO
Explanation:
Data
Silver = Ag = 0.1 mole
Nitric acid = HNO3 = 10 ml 6 M
Balanced reaction
3Ag + 4HNO₃ ⇒ 3AgNO₃ + NO + 2H₂O
# moles of HNO₃ = 6(0.01) = 0.06
Limiting reactant HNO₃ because for the amount of silver given
the reaction needs 0.133 moles of acid, and there
are only 0.06
4 mol of HNO₃ 1 mol of NO
0.06 mol of Ag x
x = (0.06 x 1) / 4
x = 0.015 mol
Ответ:
idk
Explanation:
god is the answer to everything