soccerjessie40901
25.03.2020 •
Chemistry
The total pressure for a mixture of N2O4 and NO2 is 1.5atm. If Kp = 7.1 at 25°C, calculate the partial pressure of each gas in the mixture. (HINT 1.5 = 2pNO2 + pN2O4) 2NO2 (g) = N2O4 (g)
Solved
Show answers
More tips
- S Style and Beauty Don t Sacrifice Your Brows: How to Properly Pluck Stubborn Hairs...
- W Work and Career Secrets of a Super Supervisor: Proper Team Management...
- P Photography and Videography Understanding HDR: How It Works and Why You Need It...
- G Goods and services Which TV is better - LCD or Plasma?...
- S Sport How to Learn to Pull Up on Monkey Bars?...
- L Leisure and Entertainment Scrapbooking: What is it and Why is it Becoming More Popular?...
- C Computers and Internet Where did torrents.ru move to?...
- B Business and Finance Understanding Cash Flow: What It Is and How It Works...
- C Computers and Internet What Are Peers and Seeds in Torrenting?...
- H Health and Medicine 10 Simple Techniques on How to Boost Your Mood...
Answers on questions: Chemistry
- C Chemistry Ok those are the questions Which of the following is the smallest particle of matter? Atom, cell , molecule, organ What will most likely happen in the absence of a nucleus?...
- C Chemistry A 2.77 g lead weight, initially at 11.1 oC, is submerged in 7.94 g of water at 52.8 oC in an insulated container. clead = 0.128 J/goC; cwater = 4.18 J/goC. What is the...
- C Chemistry Which is an example of a chemical reaction...
- C Chemistry 1. What type of heat transfer occurs as you stick your hand into a warm pool and you can feel the heat of the water? 2.What type of heat transfer occurs when a heating...
- C Chemistry If 5 moles of sugar was added to a kilogram of water, the new boiling point would be degrees C. Write your answer to the nearest hundredth....
- C Chemistry CH₄ + O₂ → H₂O + CO₂ , ¿Cuántos gramos de H₂O se producen?...
- C Chemistry Express the chemical reaction between hydrogen and oxygen elements for the formation of water in word equation and chemical equation(in short)...
- B Biology What is associated with the wind star...
- S Social Studies Which of the following statements fits BEST in the space labeled 3?...
- M Mathematics 3: Please help. Circle C has its center at (9,0) and a point is on the circle at A(8,6). Which answer verifies whether point P(10,−7) lies on the circle?...

Ответ:
Explanation:
In order to calculate the partial pressure for each gas , all you need to do is multiply the mole fraction of each gas with the total pressure.
It is given that = 1.5 atm
= 1.5 atm
The mole fraction of each gas can be calculated in the following way:
Partial pressure of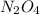 : 1.5 ×
: 1.5 ×  = 0.5 atm
= 0.5 atm
Partial pressure of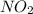 : 1.5 ×
: 1.5 ×  = 1 atm
= 1 atm
Hope that answers the question, have a great day!
Ответ:
(Answer) The name of the compound with formula N2Cl4 is dinitrogen tetrachloride.
In this compound, two central nitrogen atoms are bonded by covalent bonds by sharing valence electron pairs among each other. Two chlorine atoms are attached to each nitrogen atom by covalent bond formation.
One molecule of the compound N2Cl4 has 2 nitrogen atoms and 4 chlorine atoms. For this reason, its name is dinitrogen tetrachloride. Here, "di" represents "two" and "tetra" represents "four".