cragajjar2
04.03.2020 •
Chemistry
Uppose you perform a titration of an unknown weak acid solution. You start with 4.00 mL of the weak acid and find that it takes 12.8 mL of 0.0500 M NaOH to reach the equivalence point. What is the concentration of the unknown weak acid solution?
Solved
Show answers
More tips
- G Goods and services LED-подсветка в LCD-телевизорах: 5 причин, почему она лучше других технологий...
- P Photography and Videography Understanding HDR: How It Works and Why You Need It...
- P Photography and Videography How to Choose the Perfect Photo Paper for Your Images?...
- C Computers and Internet How to Choose an Uninterruptible Power Supply (UPS) for Your Computer: Expert Tips...
- S Science and Technology How to choose a home theater system?...
- A Auto and Moto How to Choose a Car Wash? Tips and Recommendations...
- A Animals and plants How ants survive winter: exploring the secrets of their winter life...
- C Construction and repair How to Choose the Best Underfloor Heating?...
- S Sport When is the Champions League final?...
- S Sport When and Where Will the 2014 World Cup be Held?...
Answers on questions: Chemistry
- C Chemistry Chemistry hw problem! need answer! write the equation for the ionization of hcho, (methanoic acid, commonly called formic acid) in water using a double arrow. formic...
- C Chemistry If equilibrium concentrations of nh4 and oh- are each 2 x 10^-3 m and the concentration of nh3 is 0.2 m, what is kb for ammonia?...
- C Chemistry Arrange the sequence of reactions that occur in the conversion of pyruvate to acetyl-coa in the correct order: the anion of the hydroxyethyl group attacks one sulfur...
- C Chemistry Determine the number of bonding electrons and the number of nonbonding electrons in the structure of cs2. enter the number of bonding electrons followed by the number...
- C Chemistry 10 points! ! the ionization energies of hydrogen (h), nitrogen (n), flourine (f), and oxygen (o) are 1,312, 1,402, 1742, and 1,314, respectively. which element combination...
- C Chemistry I need help I’ll give you brainliest...
- M Mathematics The Good Fruit stand sells baskets of cherries for $4 and baskets of blueberries for $3. Its daily sales goal is $720....
- H History Which phrase best captures the idea of Jacksonian Democracy ? O three branches with equal strength O against slavery and for Native Americans Ostates rights above...
- M Mathematics 6. The total cost of materials to build the foam pit is $1,285.20. What is the cost of materials per square foot?...
- S Social Studies Would You Rather Have Endless Money Or Endless Love?...

Ответ:
Explanation:
It is known that at the equivalence point,
Equivalent of acid = equivalent of base ......... (A)
and, Equivalent =
Therefore, equivalent of acid is calculated as follows.
Equivalent of acid =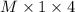 .......... (1)
.......... (1)
Equivalent of base = ............ (2)
............ (2)
Hence, using equation (A) we will calculate the concentration as follows.
M = 0.16 M
Thus, we can conclude that concentration of the unknown weak acid solution 0.16 M.
Ответ:
% is best for pie chart
explanation: