Which of the following statements is TRUE?a. The rate constant does not depend on the activation energy for a reaction where the products are lower in energy than the reactants. b. The addition of a homogeneous catalyst does not change the activation energy of a given reaction. c. Rate constants are temperature dependent. d. None of the above is true. e. A catalyst raises the activation energy of a reaction.
Solved
Show answers
More tips
- F Food and Cooking How to cook crayfish? Everything you need to know...
- G Goods and services LED-подсветка в LCD-телевизорах: 5 причин, почему она лучше других технологий...
- P Photography and Videography Understanding HDR: How It Works and Why You Need It...
- P Photography and Videography How to Choose the Perfect Photo Paper for Your Images?...
- C Computers and Internet How to Choose an Uninterruptible Power Supply (UPS) for Your Computer: Expert Tips...
- S Science and Technology How to choose a home theater system?...
- A Auto and Moto How to Choose a Car Wash? Tips and Recommendations...
- A Animals and plants How ants survive winter: exploring the secrets of their winter life...
- C Construction and repair How to Choose the Best Underfloor Heating?...
- S Sport When is the Champions League final?...
Answers on questions: Chemistry
- C Chemistry Chains of amino acids make which can join together to make a...
- C Chemistry Total the mass on the syringe record it in the correct row of the data table done click and drag weights to change the pressure. click the syringe to zoom in and...
- C Chemistry An investigation involves determining which metal is better for making pots that will cook food faster. which is the best hypothesis to use for this investigation?...
- C Chemistry What is meant by polimer...
- C Chemistry Wut is answer pls???...
- P Physics The stride length of a particular runner is 2.5 m. How many strides must that runner take to complete a 100 m race? a. 40 b. 100 c. 250 d. not enough information...
- B Business The lessons or skills that a person gains by working in a specific job or occupation is known as . A. training B. experience C. certification D. education...
- B Biology Which of the following states that submicroscopic particles of all matter are in constant random motion?...
- S Social Studies For a survey to be useful to a researcher, it must be representative of the population from which it is drawn. True or False...
- E English What is one (school appropriate) word that has been created by public figures in the last few years? What does it mean?...

Ответ:
The answer to the question which of the following statements is TRUE is
c. Rate constants are temperature dependent.
Explanation:
The rate constant can be derived from the following Arrhenius equation thus
k =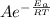
Where A = Arrhenius constant
R = Universal gas constant
T= Absolute temperature
Eₐ = Activation energy of the reaction
The above equation makes it clear that the rate of a reaction is mainly impacted by the temperature of the reaction.
The rate constant therefore is not actually a constant as it is only valid for a specified temperature
Ответ:
here is a answer
Explanation:
The green alternative
Unlike fossil fuels, green energy made from wind and solar power is sustainable, because its generated by resources that won't run out.
please mark me as brainlist and follow me