ddmoorehouseov75lc
30.07.2019 •
Chemistry
Why does water (h2o) have a significantly higher boiling point than methane (ch4)? hydrogen forms a stronger covalent bond with oxygen than with carbon. hydrogen forms a weaker covalent bond with oxygen than with carbon. water molecules are attracted to one another by hydrogen bonds. water molecules are made of fewer atoms than methane molecules.
Solved
Show answers
More tips
- A Art and Culture Who Said The Less We Love a Woman, the More She Likes Us ?...
- F Family and Home How to Get Rid of Your Neighbors?...
- S Society and Politics How Could Nobody Know About the Dead Mountaineers?...
- H Health and Medicine How to Cure Adenoids?...
- H Health and Medicine Why Wearing a Back Brace Can Be Beneficial During Back Strain?...
- S Sport When and Where Will the 2014 World Cup be Held?...
- C Computers and Internet How to Choose a Monitor?...
- H Horoscopes, Magic, Divination Where Did Tarot Cards Come From?...
- S Style and Beauty How to Make Your Lips Fuller? Ideas and Tips for Beautiful Lips...
Answers on questions: Chemistry
- M Mathematics What is g if negative 4/3 is equal to g over 1/2...
- P Physics A woman lives on the eighth floor of an apartment building. She works in a high-rise office building 6.5 blocks away from her apartment on the same street. Her office...
- H History In which decade did the first space shuttle launch? a. 1950s b. 1960s c. 1980s d. 1990s...
- M Mathematics What is 25% of 30? pls help me...

Ответ:
The attraction of inter-molecular forces between molecules is defined by a general term "Van der Waals forces". It is the weak interactions caused by momentary changes in electron density in a molecule.
Inter-molecular forces that are present between hydrogen atom which is bonded to highly electronegative atom ( ) and the lone pair electrons present in the other molecule on these electronegative atoms is known as hydrogen bonding.
) and the lone pair electrons present in the other molecule on these electronegative atoms is known as hydrogen bonding.
The type of interactions between methane, is Van der Waals interactions whereas in water,
is Van der Waals interactions whereas in water, 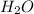 is hydrogen bonding.
is hydrogen bonding.
Since, the hydrogen bond is stronger than the Van der Waals forces so, it results in higher strength between the molecule possessing hydrogen bonding. Thus, molecules possessing hydrogen bonds will have higher boiling point than the molecules which possess Van der Waals forces.
Hence, water (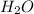 ) have a significantly higher boiling point than methane (
) have a significantly higher boiling point than methane (  ) because water molecules are attracted to one another by hydrogen bonds.
) because water molecules are attracted to one another by hydrogen bonds.
Ответ:
answer:
step-by-step explanation: