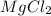dontcareanyonemo
29.03.2021 •
Chemistry
WILL GIVE BRANLIEST
1)how many liters of HCl are produced when 47.2 L of chlorine are reacted with excess hydrogen at STP
2)if you need to make 24.0 g LiOH , how many grams of Li3N must you react with excess water?
3)How many moles of hydrogen gas can be produced from 13.2 moles of hydrochloric acid (HCl)
4)if 34.9g of copper (ll) chloride reacts with 42.1g of sodium nitrate what is the limiting reactant?
Solved
Show answers
More tips
- A Art and Culture The History and Characteristics of Jazz Bands: A Deep Dive...
- F Food and Cooking From Latte to Espresso: Which Coffee Drink is the Most Popular on Earth?...
- C Computers and Internet How to Set Up Internet on iPhone? Detailed Guide with Step-by-Step Instructions...
- P Philosophy 8 привычек, чтобы достичь счастливой жизни...
- F Food and Cooking How Many Grams Are In a Tablespoon?...
- G Goods and services How to Choose the Right Iron: Purchase Tips...
- S Style and Beauty How to Choose the Perfect Hair Straightener?...
- H Health and Medicine How to Choose the Right Glasses?...
- H Health and Medicine What vaccines do children need?...
- H Health and Medicine AKDS Vaccination: Ensure Your Child s Safety...
Answers on questions: Chemistry
- M Mathematics I will love and rate 5.0 if done correctly with no images and no trolling for answer. A hardware store receives shipments containing 600 light bulbs each. A sample of 75 light bulbs...
- M Mathematics HELP I WILL GIVE 50 POINTS HURRY PLZ Classify each pair of numbered angles as corresponding, alternate interior, alternate exterior, or none of these. Drag and drop the choices into...
- M Mathematics Garrett is 3 years older than Rachael. In 2 years the sum of their ages will be 39. How old is Garrett now? PLEASE HELP THANKSSS...
- B Business the members of a marketing department are having a meeting. there is a lot of disagreement over the content of the next advertisement. this scenario best illustrates the level of...

Ответ:
Ответ:
(i) 0.261 moles of HCl, (ii) 0.131 moles of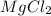 and (iii) 12.5 grams of
and (iii) 12.5 grams of 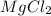
Explanation: The balanced equation for the formation of HCl by the reaction magnesium chloride with water is:
From above balanced equation, there is 1:2 mol ratio between magnesium chloride and HCl.
As per the given information, 5.85 L of HCl gas is produced. here, temperature and pressure is not mentioned, so let's assume STP conditions.
At STP, volume of 1 mol of a gas is 22.4 L. With the help of this, we can calculate the moles of HCl present in 5.85 L.
= 0.261 mol HCl
As there is 1:2 mol ratio between magnesium chloride and HCl, we can calculate the moles of magnesium chloride from the above calculated moles of HCl as:
= 0.131 mol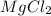
Molar mass of magnesium chloride is given as 95.2 gram per mol. On multiplying the moles by molar mass we can calculate its mass reacted.
= 12.5 g