emmaty7845
18.07.2019 •
Physics
If 0.40 moles of pcl5 is heated in a 10.0 l container, equilibrium is established in which 0.25 moles of cl2 are present. the reaction is: pcl5(g)\longleftrightarrow ⟺ pcl3(g) + cl2(g) what is the value of the equilibrium constant?
Solved
Show answers
More tips
- H Health and Medicine Is Massage Necessary? Facts and Opinions...
- C Computers and Internet Clearing Cache: How to Speed Up Your Browser...
- S Style and Beauty How are artificial nails removed?...
- S Style and Beauty Secrets of Tying a Pareo: 5 Ways...
- F Food and Cooking Everything You Need to Know About Pasta...
- C Computers and Internet How to Choose a Monitor?...
- H Horoscopes, Magic, Divination Where Did Tarot Cards Come From?...
- S Style and Beauty How to Make Your Lips Fuller? Ideas and Tips for Beautiful Lips...
Answers on questions: Physics
- P Physics 1. If your brain is big why isnt your head big? 2. If your white doesnt that make me black? 3. If fish smell bad but taste good what does poop taste like?...
- P Physics Draw the reactants using the drawing tool. Keep in mind that one molecule of nitrogen has two bonded atoms, and one molecule of hydrogen has two bonded atoms. INT File: Width:...
- P Physics What mediums do each typically travel through?...
- P Physics Student Exploration: Nuclear Decay. Has anyone done a Gizmos lab on this?...
- P Physics The bumper cars crash into each other and stop. Explain why both bumper cars stop after the crash. [4marks]...
- G Geography What happens to rock when acid reacts with it?...
- M Mathematics As a salesperson you are pay $50 per week plus $2 for sale this week you want your pee to be at least $100 what is the minimum number of sales you must make to earn at least...
- G Geography Where is the world’s shortest commercial runway?...
- H History Although the lustitania was sunk in 1915,and it angered many american, the government refused to goto war. why do you think the country remained out of the war for two more...
- M Mathematics Aplumber had a pipe that was 2 \text { yards}2 yards long. he cut 18 \text{ inches}18 inches off the end of the pipe. how many feet long was the pipe after the plumber cut it?...

Ответ:
0.0036
Explanation:
Initial moles of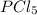 = 2 mole
= 2 mole
Moles of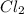 at equilibrium= 0.25 mole
at equilibrium= 0.25 mole
Volume of container = 10 L
Initial concentration of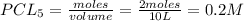
equilibrium concentration of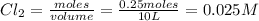
The given balanced equilibrium reaction is,
Initial conc. 0.2 M 0 0
At eqm. conc. (0.2-x) M xM xM
The expression for equilibrium constant for this reaction will be,
We are given : x = 0.025 M
Now put all the given values in this expression, we get :
Thus the value of the equilibrium constant is 0.0036.
Ответ:
Explanation :
When a ray of light passes through the narrow opening then the light diffracts. The process is known as the diffraction of light.
By examining a wave's pattern after it interacts with a barrier or gap, measurements can be made to better understand diffraction wave phenomena.
After diffracting the light forms a pattern consisting of dark and bright bands.
Hence, the question could be " How diffraction of wave occurs? Explain ".