rileyeddins1010
31.07.2019 •
Physics
Phosphorous reacts with chlorine gas to produce phosphorous pentachloride. calculate the mass of product produced when 25.0 g of phosphorous reacts with 25.0 grams of chlorine. calculate the mass of product produced if the reaction occurred with a 70.5 percent yield.
Solved
Show answers
More tips
- C Cities and Countries Which Country has the Most Expensive Visa?...
- F Family and Home Tender Care for Your Parquet: Is it Possible to Clean Parquet?...
- S Society and Politics Is It Fact or Fiction? Let s Talk About Anton Chekhov s Pseudonym...
- S Sport Playing Bowling: Rules and Advice for Novices...
- C Computers and Internet How to Properly Repartition a Hard Drive?...
- A Auto and Moto What Is the Cost of Customs Clearance for a Car in Russia?...
- L Leisure and Entertainment Should You Buy a Ceramic Knife?...
- C Computers and Internet How to easily and quickly disable Firebug in Gmail and Google Docs...
- G Goods and services How to sew a ribbon: Tips for beginners...
- F Food and Cooking How to Make Mayonnaise at Home? Secrets of Homemade Mayonnaise...
Answers on questions: Physics
- P Physics What determines the volume of a gas? o temperature and gravity o temperature and pressure only pressure o only temperature...
- P Physics An elements properties can be predicted from its...
- P Physics The process by which electrons are transferred from a neutral object to a nearby charged object, without touching? -Friction -Conduction -Induction -Convection...
- P Physics Unblanced forces cause:reversalinertiamotiongravity...
- P Physics Urgent ! a spring has a spring constant of 105 n/m. if you compress the spring 0.1 m past its natural length, what force does the spring apply? a. 500n, b. 10.5n, c. 0.0005n,...
- P Physics Does ionic bonds loose or gain electrons ?...
- P Physics List some exothermic and endothermic processes...
- P Physics The speed of light is now defined to be c-2.99792458 x 10^8 m/s...
- P Physics Meredith notices that different plant fertilizers are made up of different chemicals. some fertilizers are high in nitrogen. other fertilizers are high in phosphorus. meredith...
- P Physics An astronaut weighs 700 n on earth what is the astronaut s mass on earth?...

Ответ:
Answer : The mass of product produced if the reaction occurred with a 70.5 percent yield will be, 20.67 grams.
Explanation : Given,
Mass of P = 25 g
Mass of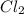 = 25 g
= 25 g
Molar mass of P = 30.97 g/mole
Molar mass of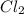 = 71 g/mole
= 71 g/mole
Molar mass of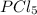 = 208.24 g/mole
= 208.24 g/mole
First we have to calculate the moles of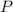 and
and 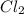 .
.
Now we have to calculate the limiting and excess reagent.
The balanced chemical reaction is,
From the balanced reaction we conclude that
As, 5 moles of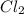 react with 2 moles of
react with 2 moles of 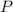
So, 0.352 moles of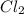 react with
react with 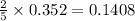 moles of
moles of 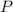
That means, in the given balanced reaction,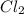 is a limiting reagent and it limits the formation of products and
is a limiting reagent and it limits the formation of products and 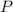 is an excess reagent because the given moles are more than the required moles.
is an excess reagent because the given moles are more than the required moles.
Now we have to calculate the moles of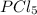 .
.
As, 5 moles of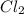 react with 2 moles of
react with 2 moles of 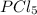
So, 0.352 moles of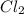 react with
react with 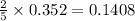 moles of
moles of 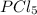
Now we have to calculate the mass of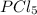 .
.
Now we have to calculate the mass of product produced (actual yield).
Therefore, the mass of product produced if the reaction occurred with a 70.5 percent yield will be, 20.67 grams.
Ответ:
1) Yes
2)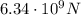
Explanation:
1)
To solve this part, we have to calculate the pressure at the depth of the batyscaphe, and compare it with the maximum pressure that it can withstand.
The pressure exerted by a column of fluid of height h is:
where
h is the height of the column of fluid
Here we have:
h = 5440 m is the depth at which the bathyscaphe is located
Therefore, the pressure on it is
Since the maximum pressure it can withstand is 60 MPa, then yes, the bathyscaphe can withstand it.
2)
Here we want to find the force exerted on the bathyscaphe.
The relationship between force and pressure on a surface is:
where
p is hte pressure
F is the force
A is the area of the surface
Here we have:
The bathyscaphe has a spherical surface of radius
r = 3 m
So its surface is:
Therefore, we can find the force exerted on it by re-arranging the previous equation: