aaronjin4443
03.02.2020 •
Chemistry
1. chemical equilibrium is established when the number of reactants equals the number of products.. - true. - false. 2. according to le chatelier's principle, by increasing the temperature of the system shown below, the equilibrium will shift to the right (towards . a + b heat + ab. - true. - false. 3. for which of the following reactions will an increase in pressure not effect the position of equilibrium? . . a. 2a2 (g) + b2 (g) 2a2b (g). b. 2ab (g) a2 (g) + b2 (g). c. 2a (g) + f2 (g) 2af (g). d. 2b (s) + 2ha (aq) 2ba (aq) + h2 (g).
Solved
Show answers
More tips
- C Computers and Internet How to Reinstall Windows: A Detailed Guide for Beginners...
- S Style and Beauty How to Get Rid of a Bruise: Tips and Tricks...
- F Food and Cooking Лечо: вкусное и простое блюдо для любой кухни...
- H Health and Medicine Relieving Swelling in Legs: Causes and Ways to Alleviate the Symptom...

Ответ:
1. False
2. False
3.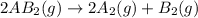
Explanation:
1. Chemical equilibrium are attained is closed system.
The macroscopic properties remain constant like: volume, pressure, energy etc. Rate of forward reaction is equal to the rate of backward reaction.
The concentration of the reactants and products remain constant.They are not always equal.
2.
By increasing the temperature:
If the temperature is increased, so according to the Le-Chatelier's principle , the equilibrium will shift in the direction where decrease in temperature occurs. As, this is an exothermic reaction, backward reaction will decrease the temperature. Hence, the equilibrium will shift in the left direction (i.e. towards reactants.)
3. If the pressure of the container is increased, the pressure will decrease according to Boyle's Law. Now, according to the Le-Chatelier's principle, the equilibrium will not shift in any direction when the number of moles of gas molecules is equal at the product side and the reactant side.
Ответ:
C) f(-3) = g(-3)
Step-by-step explanation:
This is the answer because the lines intersect at the point (-3, -4). When you plug in the x-value of -3 into both equations, the y-value will be equal.