williamslyric
30.04.2021 •
Chemistry
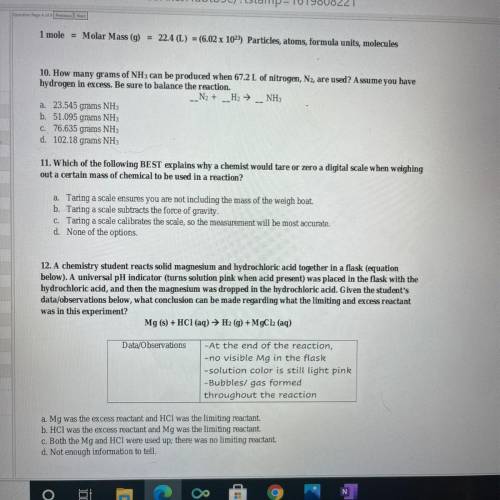
10. How many grams of NH 3 can be produced when 67.2 L of nitrogen, , are used? Assume you have N 2 hydrogen in excess. Be sure to balance the reaction. 12. A chemistry student reacts solid magnesium and hydrochloric acid together in a flask (equation below). A universal pH indicator (turns solution pink when acid present) was placed in the flask with the hydrochloric acid, and then the magnesium was dropped in the hydrochloric acid. Given the student's data/ observations below, what conclusion can be made regarding what the limiting and excess reactant was in this experiment?
Solved
Show answers
More tips
- D Dating, Love, Relationships How Long Can Love Last?...
- S Science and Technology How to Make a Homemade Smoker: The Ultimate Guide...
- S Society and Politics 10 Tips for Boosting Your Self-Esteem...
- C Computers and Internet How to Create a Folder on Your iPhone?...
- G Goods and services How to sew a ribbon: Tips for beginners...
- F Food and Cooking How to Make Mayonnaise at Home? Secrets of Homemade Mayonnaise...
- C Computers and Internet Which Phone is Best for Internet Surfing?...
- F Food and Cooking Everything You Need to Know About Pasta...
- C Computers and Internet How to Choose a Monitor?...
- H Horoscopes, Magic, Divination Where Did Tarot Cards Come From?...
Answers on questions: Chemistry
- C Chemistry Can someone do this? Without just giving me a link....
- C Chemistry Substances have a fixed arrangement of atoms. true or false (NO LINKS PLEASE)...
- C Chemistry What is the molarity of the resulting solution when 300. mL of a 0.400 M solution is diluted to 800. mL?...
- C Chemistry Anyone know how to do this? It’s for Chemisty...
- C Chemistry Zinc chloride (ZnCl2) is used as a flux when joining metals. It also is used to fireproof and remove odor from clothing. Reacting the metal zinc (Zn) with hydrochloric...
- C Chemistry What is nuclear fusion? Write a paragraph....
- C Chemistry Calculate the ph of a 0.10 m solution of hypochlorous acid, hocl. ka of hocl is 3.5×10−8 at 25 ∘c. express your answer numerically using two decimal places....
- M Mathematics Find the directional derivative of f at the given point in the direction indicated by the angle θ. f(x, y) = 8x + 9y , (9, 1), θ = −π/6...
- S Spanish Llena el espacio en blanco con la conjugación correcta del verbo en paréntesis. que te a0 (sentir) mejor. translation: fill in the blank with the correct conjugation...
- H History The slave trade never existed before america was discovered. true or false?...

Ответ: