tmoneytj28
28.11.2020 •
Chemistry
2. HI(g) → H2(g) + I2(g)
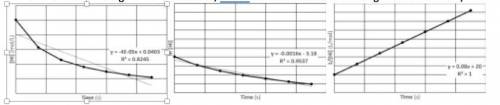
A 0.050 M sample of hydrogen iodide is decomposed into hydrogen and iodine, according to the equation above. The [HI], ln[HI], and 1/[HI] vs. time graphs are plotted. (The equations are given for the best-fit straight line for the data, and R2 value indicates the strength of correlation.)
a. Write the rate law for this reaction. Justify your response using the graphs provided.
b. Find the value of the rate constant, k. Include units.
c. At what time will [HI] be 0.001 M?
image of graphs are attached
Solved
Show answers
More tips
- F Family and Home Stay Warm but Don t Overheat: What is the Optimal Temperature for Your Home During Winter?...
- H Health and Medicine How to Treat the Flu: A Comprehensive Guide...
- O Other What is a Disk Emulsifier and How Does it Work?...
- H Health and Medicine How to Calm Your Nerves? Expert Tips That Actually Work...
- A Animals and plants 5 Tips for Taking Care of Yews to Keep Them Green and Beautiful...
- S Sport How to wrap boxing hand wraps? Everything you need to know!...
- F Food and Cooking 10 Reasons Why You Should Avoid Giving Re-Gifts: An Informative Guide...
- F Family and Home Tender Care for Your Parquet: Is it Possible to Clean Parquet?...
- S Style and Beauty How Are Eyelash Extensions Applied? All Your Questions Answered...
- F Food and Cooking 10 Tips for Proper Sushi Consumption...
Answers on questions: Chemistry
- C Chemistry List a functional group vibration that provides unambiguous evidence that the desired alcohol product was isolated. Answer using the format, for example, ketone C=O...
- C Chemistry N2(g) + 3H2(g) ⇄ 2NH3(g) ΔH = -92kJmol-1 Consider the chemical reaction for the manufacture of ammonia in the Haber process. The ΔH value shows that the reaction...
- C Chemistry I NEED HELP ASAP I WILL GIVE Is a chemical reaction taking place during Xavier’s experiment? How do you know?...
- C Chemistry How many atoms are in C6H4Cl2 (l is an L)...
- C Chemistry PLEASE HURRY Which of the following describes how the San Andreas Fault formed on Earth at a transformed boundary? Plates moved in closer Plates moved away from each...
- S Social Studies What is NOT one of the rights guaranteed by the BILL OF RIGHTS (Amendments 1-10) * Individual Freedoms Voting Rights Protections Against Government Abuse and Power...
- M Mathematics 20. In the 7th grade, there are 84 students who are either gymnasts or hockey players. 24 of the students are gymnasts, and the rest are hockey players. Which statement...
- E English The canoe is mentioned quite often. What is it a symbol for?...
- E English How is Malala powerful...
- M Mathematics Prove that the medians to the legs of an isosceles triangle are congruent....

Ответ:
Hakluyt's arguments that colonization of the Americas would be a boon to English commerce and an opportunity to Christianize the Virginia Indians likely influenced the views of his cousin, who gave them wider currency.
Explanation: