A 20.00 mL Ba ( OH ) 2 solution of unknown concentration was neutralized by the addition of 42.50 mL of a 0.1255 M HCl solution. Write the balanced molecular equation for the neutralization reaction between HCl and Ba ( OH ) 2 in aqueous solution. Include physical states. molecular equation: Ba^{2+}(aq) +2OH^{-}(aq) +H^{+}(aq) +Cl^{-}(aq)<=>H_{2}O(l) +Ba^{2+}(aq) +Cl^{-}(aq) Ba 2 + ( aq ) + 2 OH − ( aq ) + H + ( aq ) + Cl − ( aq ) − ⇀ ↽ − H 2 O ( l ) + Ba 2 + ( aq ) + Cl − ( aq ) Calculate the concentration of Ba ( OH ) 2 in the original 20.00 mL solution. [ Ba ( OH ) 2 ] = M Calculate the concentrations of Ba 2 + and Cl − in solution following the neutralization reaction.
Solved
Show answers
More tips
- C Computers and Internet What is the Meaning of lol and How Did it Become Popular?...
- F Food and Cooking What s the Best Rice for Cooking Plov?...
- F Family and Home How to Remove Fading from Clothes: Tips and Tricks...
- F Food and Cooking How to Make Polendwitsa at Home?...
- F Family and Home Parents or Environment: Who Has the Most Influence on a Child s Upbringing?...
- P Philosophy Unbelievable stories of encounters with otherworldly forces...
- L Leisure and Entertainment How to Choose the Perfect Gift for Men on February 23rd?...
- H Health and Medicine How to Treat Whooping Cough in Children?...
- H Health and Medicine Simple Ways to Lower Cholesterol in the Blood: Tips and Tricks...
- O Other How to Choose the Best Answer to Your Question on The Grand Question ?...
Answers on questions: Chemistry
- C Chemistry How many individual stands is dna composed of...
- C Chemistry Please please help me please please help please please help me please please help please please help me please...
- C Chemistry What is an equilibrium constant? A. A constant relating the effects of activation energy and temperature on the reaction rate B. The value of the rate of the forward and...
- C Chemistry The pH of pool water is normally kept between 7.2 and 7.6. Is the pool water acidic, basic or neutral? Why?...
- C Chemistry + = positive - = negative + 누 XX + + What will happen to the two objects in the picture as they are slowly moved toward each other?...
- C Chemistry If a had a number that was 12.47, what would be the answer rounded to 2 sig figs?...
- C Chemistry Ithink its c but i m not sure more missions have been flown to this planet than any other place in the solar system except the moon. many of these unmanned missions have...
- C Chemistry A student makes a solution of sulfuric acid, H2SO4, by dissolving 345.8 grams of H,So, in 575 mL solution. Calculate the molarity of the solution....
- C Chemistry You are preparing a solution of lithium bromide to use during an experiment. The experiment calls for 909.7 mL a 18 M solution. How many moles of the solute must be used...
- C Chemistry Give the formula for the alkyne containing 40 hydrogens....

Ответ:
The concentration of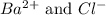 ions in the solution are 0.0426 M and 0.0852 M respectively
ions in the solution are 0.0426 M and 0.0852 M respectively
Explanation:
Neutralization reaction is defined as the reaction in which an acid reacts with a base to produce a salt and water molecule.
The chemical equation for the reaction of HCl and barium hydroxide follows:
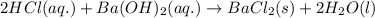
To calculate the concentration of acid, we use the equation given by neutralization reaction:where,
We are given:
Putting values in above equation, we get:
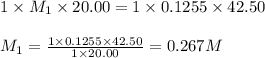
To calculate the number of moles for given molarity, we use the equation:Molarity of HCl solution = 0.1255 M
Volume of solution = 42.50 mL = 0.04250 L (Conversion factor: 1 L = 1000 mL)
Putting values in equation 1, we get:
By Stoichiometry of the reaction:
2 moles of HCl produces 1 mole of barium chloride
So,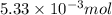 of HCl will produce =
of HCl will produce = 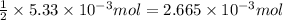
Calculating the concentration of barium chloride by using equation 1:Moles of barium chloride =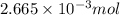
Volume of solution = [20.00 + 42.50] mL = 62.5 mL = 0.0625 L
Putting values in equation 1, we get:
1 mole of barium chloride produces 1 mole of barium ions and 2 moles of chloride ions
So, concentration of barium ions = 0.0426 M
Concentration of chloride ions = (2 × 0.0426) = 0.0852 M
Hence, the concentration of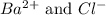 ions in the solution are 0.0426 M and 0.0852 M respectively
ions in the solution are 0.0426 M and 0.0852 M respectively
Ответ:
t6666666666644444444444444444444444
Explanation: