30valgolden
03.12.2020 •
Chemistry
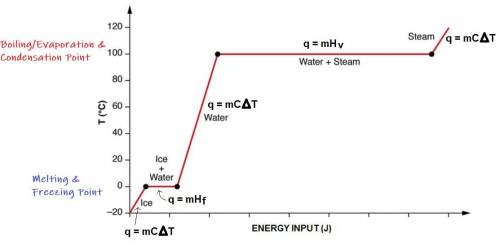
A 200 g sample of water at 60.0 degrees Celsius is heated to water vapor at 140.0 degrees Celsius. Expected Answer = 501,440 J Before trying to solve this problem, explain:
what is happening to the water from 60.0 degrees Celsius to 100.0 degrees Celsius?
what happens at 100.0 degrees Celsius?
what happens from 100.0 degrees Celsius to 140.0 degrees Celsius?
Then solve the full problem, showing work & units.
Solved
Show answers
More tips
- H Health and Medicine Coughing: Causes, Types, and Treatment Methods...
- H Health and Medicine How to Treat the Flu: A Comprehensive Guide...
- O Other What is a Disk Emulsifier and How Does it Work?...
- H Health and Medicine How to Calm Your Nerves? Expert Tips That Actually Work...
- A Animals and plants 5 Tips for Taking Care of Yews to Keep Them Green and Beautiful...
- S Sport How to wrap boxing hand wraps? Everything you need to know!...
- F Food and Cooking 10 Reasons Why You Should Avoid Giving Re-Gifts: An Informative Guide...
- F Family and Home Tender Care for Your Parquet: Is it Possible to Clean Parquet?...
- S Style and Beauty How Are Eyelash Extensions Applied? All Your Questions Answered...
- F Food and Cooking 10 Tips for Proper Sushi Consumption...
Answers on questions: Chemistry
- M Mathematics We use categorical variables to determine where someone s score falls along a continuum or range of scores...
- C Chemistry If earth s axis was perpendicular to the plane of earth s orbit what would definitely be affected?...
- E English Write the question tag for the following question. 1. please come in,? 2. Let s walk a bit faster,?pls anyone ans asap...
- B Biology The tissue illustrated above is: A. Squamous epithelium B. Columnar epithelium C. Nervous D. Striated muscle E. Lymph Please answer...

Ответ:
Ответ:
Good. What about you? ..