mckinleesmomp6qj1e
10.06.2020 •
Chemistry
A 8.00g of a certain Compound X, known to be made of carbon, hydrogen and perhaps oxygen, and to have a molecular molar mass of 44./gmol, is burned completely in excess oxygen, and the mass of the products carefully measured: product mass carbon dioxide 24.01g water 13.10g Use this information to find the molecular formula of X.
Solved
Show answers
More tips
- S Style and Beauty How to Choose the Perfect Hair Straightener?...
- F Family and Home Why Having Pets at Home is Good for Your Health...
- H Health and Medicine How to perform artificial respiration?...
- H Health and Medicine 10 Tips for Avoiding Vitamin Deficiency...
- F Food and Cooking How to Properly Cook Buckwheat?...
- F Food and Cooking How Many Grams Are In a Tablespoon?...
- L Leisure and Entertainment Carving: History and Techniques for Creating Vegetable and Fruit Decorations...
- P Photography and Videography How to Choose the Perfect Photo Paper for Your Images?...
- H Health and Medicine What vaccines do children need?...
- H Health and Medicine Reasons for the Appearance of Warts: Everything You Need to Know...
Answers on questions: Chemistry
- C Chemistry What integer represents the total charge of 4 protons...
- C Chemistry What is kinetic energy?...
- C Chemistry Which balanced equation BEST describes this reaction?...
- C Chemistry Acids are substances that ionize to form free H ions in solution whereas bases are substances that combine with H ions. a. True b. False...
- C Chemistry Pb(NO3)+MgI2=Mg(NO3)+PbI2 what type of reaction?...
- C Chemistry My feet get hot as soon as I touch the sand. What kind of heat transfer occurred?...
- C Chemistry Consider the image below depicting an ideal gas contained within a 4.5 L 4.5 L cylinder under a constant force. If 3.0 × 10 − 3 moles 3.0×10−3 moles of gas are occupying...
- C Chemistry G Solid sulfur hexafluoride evaporates when heated at atmospheric pressure rather than liquefying. What is the correct name of the type of phase transition that is...
- C Chemistry Acetylene gas (C2H2) at 25 ºC, 1 atm enters a reactor operating at steady state and burns completely with 140% theoretical air entering at 400 K, 1 atm. If the products...
- C Chemistry (3x^4)^4 I need the steps to...

Ответ:
C3H6.
Explanation:
Data obtained from the question:
Mass of the compound = 8g
Mass of CO2 = 24.01g
Mass of H2O = 13.10g
Next, we shall determine the mass of C, H and O present in the compound. This is illustrated below:
Molar Mass of CO2 = 12 + (2x16) = 44g/mol
Molar Mass of H2O = (2x1) + 16 = 18g/mol
Mass of C in compound = Mass of C/Molar Mass of CO2 x 24.01
=> 12/44 x 24.01 = 6.5g
Mass of H in the compound = Mass of H/Molar Mass of H2O x 13.1
=> 2x1/18 x 13.1 = 1.5g
Mass of O in the compound = Mass of compound – (mass of C + Mass of H)
=> 8 – (6.5 + 1.5) = 0
Next, we shall determine the empirical formula of the compound. This is illustrated below:
C = 6.5g
H = 1.
Divide by their molar mass
C = 6.5/12 = 0.54
H = 1.4/1 = 1.
Divide by the smallest
C = 0.54/0.54 = 1
H = 1/0.54 = 2
Therefore, the empirical formula is CH2
Finally, we shall determine the molecular formula as follow:
The molecular formula of a compound is a multiple of the empirical formula.
Molecular formula = [CH2]n
[CH2]n = 44
[12 + (2x1)]n = 44
14n = 44
Divide both side by 14
n = 44/14
n = 3
Molecular formula = [CH2]n = [CH2]3 = C3H6
Therefore, the molecular formula of the compound is C3H6
Ответ:
92.3g
Explanation:
Given parameters:
Mass of water = 540g
Percentage by mass of solution = 15%
Unknown:
Mass of sucrose = ?
Solution:
A solution is a mixture made up of solutes dissolved in a solvent. The solvent is the liquid and the solute is usually the solid.
Solution = solute + solvent
To find the weight percent of solute in solution ;
=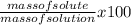
Mass of solution = mass of sucroce + water
= S + 540
where S is the mass of sucrose;
therefore;
15 =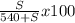
solving for S;
S = 92.3g