jean143965
20.07.2021 •
Chemistry
A chemical reaction takes place inside a flask submerged in a water bath. The water bath contains 6.90kg of water at 34.7 degrees C . During the reaction 57.1kJ of heat flows out of the bath and into the flask. Calculate the new temperature of the water bath. You can assume the specific heat capacity of water under these conditions is 4.18J.g^(-1).K^(-1) . Round your answer to significant digits.
Solved
Show answers
More tips
- G Goods and services Stock center - a modern way of optimizing logistics...
- F Food and Cooking How to Properly Wash a Down Jacket? Tips from Experts...
- C Computers and Internet Thin Client: What It Is and Why You Need It?...
- F Food and Cooking The Most Delicious and Simple Fish in Batter Recipe...
- F Food and Cooking Red Caviar: How to Choose the Best?...
- C Computers and Internet Clearing Cache: How to Speed Up Your Browser...
- S Style and Beauty How are artificial nails removed?...
- S Style and Beauty Secrets of Tying a Pareo: 5 Ways...
- F Food and Cooking Everything You Need to Know About Pasta...
Answers on questions: Chemistry
- C Chemistry Prin descompunerea termica a calcarului se obtin 2 oxizi...unul metalic iar celalalt nemetalic utilizat de plante in procesul fotosintezei. Descrietii pe scurt din punct...
- C Chemistry Calculate the number of atoms in 6g of magnesium...
- C Chemistry The price for copper was 5,04 per kg if the bell from a San Francisco cathedral had a weight of 5300lbs how much was is it worth...
- C Chemistry CH3CH = CHCH3 * A. alkane B. alkene C. alkyne...
- C Chemistry How do chemists organize information about elements? O A. By grouping the elements on the periodic table B. Through application of the atomic theory O C. Through use of...
- C Chemistry Why are models used to represent atoms? O A. Models explain new evidence. B. Models never change over time. C. Atoms are too small to see. O D. Models are completely accurate....
- C Chemistry What is the mass number of atom with 7neutron and 7 proton number...
- C Chemistry State which metal could be copper. Give a reason for your answer....
- C Chemistry 4. How do chemical processes result in thermal energy being released or absorbed? explain...
- C Chemistry How are atoms an example of diversity? A. Atoms can gain and lose protons. B. Atoms can change their properties. C. Atoms behave unpredictably. D. Atoms form millions...

Ответ:
Explanation:
Mass of Water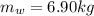
Temperature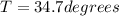
Heat Flow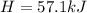
Specific heat capacity of water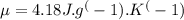
Generally the equation for Final Temperature is mathematically given by
Therefore
Ответ: