Mypasswordishotdog11
21.01.2020 •
Chemistry
A)if it takes 0.20 min to decompose 15% of a 0.300 m solution of nitrosyl chloride,what is k(the rate constant)? the decomposition of nitrosyl chloride is a second order reaction: nocl(> no(g)+1/2cl2(g)b)calculate the activation energy? when the temperature increases from 15c to 25c for a certain reaction, its rate constant doubles. calculate the activation energy, ea+ (remember: with r=8.31 j/mol.k, ea must be expressed in j/mol)
Solved
Show answers
More tips
- W Work and Career Мерчендайзинг – все, что нужно знать...
- S Science and Technology The Metric System in Our Daily Life: Understanding Its Importance...
- H Health and Medicine Angina: Causes, Symptoms, and Treatment...
- C Computers and Internet How to Learn to Type Fast?...
- F Food and Cooking Delight for Gourmets: How to Prepare Liver Pate...
- S Style and Beauty How to braid friendship bracelets?...
- H Health and Medicine Mercury Thermometer Danger: What to do when a thermometer breaks?...
- F Food and Cooking Which Calamari Salad is the Most Delicious?...
- S Society and Politics 10 Tips for Boosting Your Self-Esteem...
- F Food and Cooking The Most Delicious and Simple Fish in Batter Recipe...
Answers on questions: Chemistry
- C Chemistry 30 POINTS MATCH THE DEFINITIONS PLEASE, ITS PROBABLY EASY FOR U THO...
- C Chemistry How do total bond energy and the law of conservation of energy relate to exothermic and endothermic reactions?...
- C Chemistry Which device involves the use of plasma in technology? arc welder diesel engine a car radio battery-operated flashlight...
- C Chemistry 8.) A silver bar of metal has a volume of 5.4 cm3 and a mass of 32.6 g. Is the bar of metal aluminum? The density of aluminum is 2.7 g/cm3. a.) No, the density of this metal is...
- C Chemistry What would be the new volume be when 35 mL of helium is cooled from 285 K to 92 K?...
- C Chemistry Methane undergoes substitution reaction with chlorine. a) State the condition for this reaction.b) Name and write down the structural formula of the second substitution product....
- C Chemistry lhe energy used to power one of the Apollo lunar missions was supplied by the following overall reaction: 2N2H4 + (CH3)2N2H2 + 3N204.- 6N2 + 2C02 +81-120. For the phase of the...
- C Chemistry What is the mass of ethanol thats exacly fills a 200.0ml container?...
- C Chemistry Calculate the grams of solid product and excess reagent when 2.50 grams of barium chloride is mixed with 4.00 grams of magnesium sulfate. calculate the grams of solid product when...
- S Social Studies What strategy do parents use that allows a child to indicate an interest and then elaborate on that interest?...

Ответ:
a) k = 0.0417 M⁻¹.s⁻¹
b) Ea = 49.4 kJ/mol
Explanation:
a) If the decomposition reaction is a second-order reaction, the rate law is:
rate = k*[NOCl]²
The rate is how much the concentration decays per second (M/s), thus, if its initially 0.300 M and it decays 15%, the final concentrations 85% of 0.300, which is 0.255 M. The time needed for that is 0.20 min = 12 s, so:
rate = (0.300 - 0.255)/12
rate = 0.00375 M/s
Thus, the rate constant is:
0.00375 = k*(0.300)²
0.09k = 0.00375
k = 0.0417 M⁻¹.s⁻¹
b) By the Arrhenius equation, the constant k is related to the activation energy as:
k =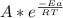
Where A is a constant, R is the gas constant (8.31 J/mol.K) and T is the temperature. If we apply ln in the equation:
ln(k) = ln(A) - Ea/RT
Thus, for T1 = 15°C (288 K), k = k1, and for T = 25°C (298 K), k = k2, knowing that Ea and A are independent of the temperature:
ln(k1) = ln(A) - Ea/RT1
ln(k2) = ln(A) - Ea/RT2
ln(k1) - ln(k2) = ln(A) - ln(A) - Ea/RT1 + Ea/RT2
ln(k1/k2) = (Ea/R) * (1/T2 - 1/T1)
If the rate constant doubles, k2 = 2k1
ln (1/2) = (Ea/8.31) * (1/298 - 1/288)
-0.6932 = (Ea/8.31)*(-1.1652x10⁻⁴)
1.402x10⁻⁵Ea = 0.6932
Ea = 49438.84 J/mol
Ea = 49.4 kJ/mol
Ответ:
Explanation: what do you need help on??? :?
Okay well it is saying you will have to give the vaccine to the patient from the shot thingy i guess