A255 ml round--bonom flask is weighed and found to have a mass of 114.85 g. a few millimeters of an easily vaporized liquid are added to the flask and the flask is immersed in a boiling water bath. all of the liquid vaporizes at the boiling tempentture of water, filling the flask with vapor. when all of the liquid has vaporized, the flask is removed from the bath, cooled, dried, and reweighed. the new mass of the flask and the condensed vapor is 115.23 g. which of the following compounds could the liquid be?
a. c₄h₁₀b. c₃h₇ohc. c₂h₆d. c₂h₅ohe. c₄h₉oh
Solved
Show answers
More tips
- C Computers and Internet Boost your processor performance with these easy tips...
- L Leisure and Entertainment When will Maslenitsa start?...
- F Food and Cooking Discovering the Mysterious Fruit of Feijoa...
- B Business and Finance How to Open an Online Store? A Detailed Guide for Beginners...
- W Work and Career How to Write a Resume That Catches the Employer s Attention?...
- C Computers and Internet Е-head: How it Simplifies Life for Users?...
- F Family and Home How to Choose the Best Diapers for Your Baby?...
- F Family and Home Parquet or laminate, which is better?...
- L Leisure and Entertainment How to Properly Wind Fishing Line onto a Reel?...
- L Leisure and Entertainment How to Make a Paper Boat in Simple Steps...
Answers on questions: Chemistry
- S Social Studies Some states hold that an engagement ring is a conditional gift that becomes an absolute (effective) gift only on marriage. other states conclude that when an engagement...
- S Social Studies President theodore roosevelt was called a trust buster because he...
- B Business Product management is responsible for what gets built as defined by the vision, roadmap, and what else? a) program backlog b) customers c) key stakeholders d) pi planning...
- M Mathematics Pls help me I do not get this T~T...

Ответ:
Option (d) is the correct answer.
Explanation:
According to the given situation, mass of compound will be calculated as follows.
Mass of compound = mass of flask and condensed vapor - mass of flask
= 115.23 - 114.85
= 0.38 g
Volume (V) = 255 mL = (as 1 ml =
(as 1 ml = 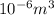 )
)
Pressure (P) = 101325 Pa
Temperature = = (100 + 273) K = 373 K
= (100 + 273) K = 373 K
Now, according to the ideal gas equation, PV = nRT
and, moles of compound n =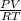
=
= 0.008332 mol
As, molar mass of compound =
=
= 46 g/mol
Therefore, the compound is (molar mass = 12 x 2 + 5 x 1 + 16 + 1 = 46 g/mol).
(molar mass = 12 x 2 + 5 x 1 + 16 + 1 = 46 g/mol).
Thus, we can conclude that out of the given options the liquid could be .
.
Ответ:
alcohol freezing is very rare as it freezes at around -114 degres