Acommon laboratory reaction is the neutralization of an acid with a base. when 32.8 ml of 0.500 m hcl at 25.0°c is added to 66.3 ml of 0.500 m naoh at 25.0°c in a coffee cup calorimeter (with a negligible heat capacity), the temperature of the mixture rises to 28.2°c. what is the heat of reaction per mole of nacl (in kj/mol)? assume the mixture has a specific heat capacity of 4.18 j/(g·k) and that the densities of the reactant solutions are both 1.07 g/ml.
Solved
Show answers
More tips
- W Work and Career How much does an honest traffic police officer earn in a day?...
- G Goods and services How to sew a ribbon: Tips for beginners...
- L Leisure and Entertainment How to Learn to Draw Graffiti: Tips for Beginners...
- F Family and Home How to Remove Tar Stains: Tips and Recommendations from Experts...
- F Family and Home How to Remove Fading from Clothes: Tips and Tricks...
- S Sport How to Do a Jumping Split...
- H Health and Medicine How Did Inna Lose Weight on Dom 2?...
- F Family and Home How to Properly Fold Napkins in a Napkin Holder?...
- F Food and Cooking How to Set Up Ventrilo - The Ultimate Guide...
- S Science and Technology How to Make a Homemade Smoker: The Ultimate Guide...
Answers on questions: Chemistry
- C Chemistry Calculate the percent composition of oxygen in pentanoic acid (C4H9COOH)....
- C Chemistry A 0.981 liter sample of NH3 is decomposed to produce how many grams of hydrogen gas? 0.0133 g 3.03 g 0.0590 g 0.0325 g...
- C Chemistry One mole of magnesium has the same number of atoms but smaller mass than one mole of sulphur true or false ...
- C Chemistry What are the three major Eras from 600 million years ago to today...
- C Chemistry Is Vastus Medialis and Lumbrical voluntary or involuntary? what muscles are biceps, triceps, deltoid, soleus, Vastus medialis, and lumbrical...
- C Chemistry If a scientist did an experiment to see how adding sugar to plant water would affect the plant’s growth, what would be the dependent variable...
- C Chemistry Definition of diameter?...
- C Chemistry Which one do you think gives the more accurate end-point pH titration or acid base titration and why?...
- C Chemistry How much heat is evolved when 1525 g of water condenses to a liquid at 100 degrees Celsius? The deltaH_condensation of water is -40.7 kJ/mol....
- C Chemistry Can some one please help me I am going to fail. I never learned how to do this please some help me. Pleaseee...

Ответ:
Explanation:
The given data is as follows.
Densities of both the reactant solutions = 1.07 g/mL.
Specific heat = 4.18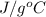
Volume of HCl = 32.8 ml
Volume of NaOH = 66.3 ml
The reaction will be as follows.
Since, density is mass divided by volume. Hence, calculate the mass of HCl as follows.
Mass of HCl = Density × Volume of HCl
=
= 35.096 g
Similarly, mass of NaOH will be calculated as follows.
Mass of NaOH = Density × Volume of NaOH
=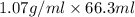
= 70.941 g
Therefore, total mass will be as follows.
Total mass = Mass of HCl + Mass of NaOH
= 35.096 g + 70.941 g
= 106.037 g
Change in temperature will be calculated as follows.
=
Therefore, calculate the heat of reaction as follows.
q =
=
= 1418.35 J
or, = 1.418 kJ (as 1 kJ = 1000 J)
No. of moles of HCl = Molarity × Volume
= 0.5 M × 32.8 ml
= 16.4 mol
No. of moles of NaOH = Molarity × Volume
= 0.5 M × 66.3 ml
= 33.15 mol
So, HCl is the limiting reagent and heat of reaction produces by per mole of NaCl will be calculated as follows.
Heat released for 1 mole of NaCl =
= 0.0864 kJ/mol
Thus, we can conclude that the heat of reaction per mole of NaCl is 0.0864 kJ/mol.
Ответ: