hardwick744
06.05.2020 •
Chemistry
An emission line of sodium has a wavelength of 330 nm. Calculate the energy of a photon of light emitted in J/ atom, and the energy emitted per mole of Na atoms at this wavelength.
Solved
Show answers
More tips
- A Auto and Moto Discovering the Leader: What is the Most Expensive Car in the World?...
- H Health and Medicine Hangover: How to Get Rid of It Quickly?...
- S Style and Beauty How to Choose the Right Fur Coat and Avoid Regrets?...
- C Computers and Internet How to Create a Folder on Your iPhone?...
- G Goods and services How to sew a ribbon: Tips for beginners...
- F Food and Cooking How to Make Mayonnaise at Home? Secrets of Homemade Mayonnaise...
- C Computers and Internet Which Phone is Best for Internet Surfing?...
- F Food and Cooking Everything You Need to Know About Pasta...
- C Computers and Internet How to Choose a Monitor?...
- H Horoscopes, Magic, Divination Where Did Tarot Cards Come From?...
Answers on questions: Chemistry
- C Chemistry Cobalt has the chemical symbol Co and the atomic number 27. How many protons, neutrons, and electrons would be found in an atom of cobalt-58?...
- C Chemistry The reaction system POBr3(g) =POBr(g) + Brz(g) is at equilibrium. Which of the following statements describes the behavior of the system if POBr is added to the container?...
- C Chemistry This is dimensional analysis so if you know it, may you help me? I will award you the brainliest. It s attached to the document below....
- C Chemistry Give the effect on the melting point of the presence of a cis double bond in a fatty acid....
- C Chemistry An unknown, naturally occurring element referred to as X exists in three isotopic forms: X-28 (27.976 amu, 92.21% abundance), X-29 (28.976 amu, 4.70% abundance), and...
- C Chemistry Which statement describes what makes the federal government different from a state government in the United States? (1 point) O The federal government is a national...
- M Mathematics | H.O.T. FOCUS ON HIGHER ORDER THINKING 14. Draw Conclusions Determine which sampling method will better represent the entire population. Justify your answer. Student...
- B Biology Energy used organisms is called?...
- E English Words to include in your writing: Congress Three Branches of Government President Colonist Britain Revolutionary War Patriot Declaration of Independence Bill of Rights...
- M Mathematics Exploring Exponential Functions: y = bx, o b 1 Complete the table of values for the function. f(x)=(1/3)^x X F(x) -2 A -1 B 0 C 1 1/3 1 1/9...

Ответ:
The energy of the photon of light emitted is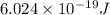 and energy emitted per mole of sodium atoms is
and energy emitted per mole of sodium atoms is 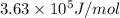
Explanation:
The relation between energy and wavelength of light is given by Planck's equation, which is:
where,
E = energy of the photon
h = Planck's constant =
c = speed of light =
Putting values in above equation, we get:
To calculate the energy emitted per mole of sodium atom, we use the equation given by Planck's equation, which is:
where,
E = energy of the photon
h = Planck's constant =
c = speed of light =
Putting values in above equation, we get:
Hence, the energy of the photon of light emitted is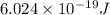 and energy emitted per mole of sodium atoms is
and energy emitted per mole of sodium atoms is 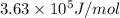
Ответ: