mackenziann
27.01.2021 •
Chemistry
An unknown clear liquid
solvent is tested for identification in the lab using
solubility tests. The unknown solvent is either methanol (CH3OH), methane
(CH4), or pentane (C5H12). The unknown liquid is mixed with the following
solutes, NaCl, H20 and C2H6. The solutes that are soluble in the unknown
solvent are NaCl and H20. What is the identification of the solvent?
Methane
Pentane
None are possible
Methanol
Solved
Show answers
More tips
- F Food and Cooking How to Make Thin Pancakes: Recipe and Tips...
- S Style and Beauty Is Hot Scissor Haircutting Beneficial or Dangerous?...
- S Style and Beauty How to Get Rid of Under Eye Bruises?...
- F Food and Cooking Is Bacon Good for You?...
- S Style and Beauty Discover the Art of Nail Design: How Do You Paint Your Nails?...
- P Philosophy How to Develop Extrasensory Abilities?...
- O Other Everything You Need to Know About Kudyabliks...
- C Computers and Internet The Twitter Phenomenon: What it is and How to Use it...
- C Computers and Internet How to Choose a Laptop: Expert Guide and Tips...
- C Computers and Internet How to Choose a Monitor?...
Answers on questions: Chemistry
- C Chemistry Enter the balanced complete ionic equation for NH4Cl(aq)+NaOH(aq)→H2O(l)+NH3(g)+NaCl(aq) . Express your answer as a chemical equation. Identify all of the phases...
- S Spanish What is la nina in spanish...
- M Mathematics You are working as an apprentice for the bksb Newcastle Arena. An indoor sport exhibition is coming to the arena. Your supervisor has asked you to help set up a...
- E English What is the length of a typical thesis statement?...
- S Social Studies Satirical arguments offer real solutions to real problems. a. true b. false...

Ответ:
6g/mL
Explanation:
Given parameters;
Mass of the unknown substance = 18g
Volume of water before the mass is introduced = 5mL
Volume of water after the mass is introduced = 8mL
Unknown:
Density of the mass = ?
Solution:
Density is the mass per unit volume of a substance.
Density =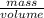
Mass is the amount of matter in a substance
Volume is the space occupied by a body
Volume of the body = 8mL - 5mL = 3mL
Insert the parameters and solve;
Density =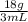 = 6g/mL
= 6g/mL