1230bering
15.04.2020 •
Chemistry
Analysis of a sample of a gaseous compound shows that it contains 85.7% C and 14.3% H by mass. At standard conditions, 112 mL of the gaseous compound weighs 0.21 g. What is the molecular formula for the compound
Solved
Show answers
More tips
- F Food and Cooking What age is appropriate for giving your child cocoa?...
- A Auto and Moto How to Start a Diesel Engine in Cold Weather?...
- F Family and Home How to Remove Tar Stains: Tips and Recommendations from Experts...
- F Family and Home How to Remove Fading from Clothes: Tips and Tricks...
- S Sport How to Do a Jumping Split...
- H Health and Medicine How Did Inna Lose Weight on Dom 2?...
- F Family and Home How to Properly Fold Napkins in a Napkin Holder?...
- F Food and Cooking How to Set Up Ventrilo - The Ultimate Guide...
- S Science and Technology How to Make a Homemade Smoker: The Ultimate Guide...
- A Auto and Moto Battle for the Relocation of The Cherkizovsky Market: Who Won?...
Answers on questions: Chemistry
- C Chemistry the range of pressure in Earth’s interior where rock with a density range of 9.9 to 12.2 g/cm3 is found...
- C Chemistry Who wants a girlfriend best answer gets brainliest...
- C Chemistry In the laboratory you dilute 2.49 ml of a concentrated 6.00 m hydrochloric acid solution to a total volume of 50.0 ml. what is the concentration of the dilute solution?...
- C Chemistry In the presence of an emulsifying agent, a mixture of oil and water becomes a...
- C Chemistry The atomic number of carbon is 6. how many electrons does an atom of carbon have if it is represented by the symbol shown below? 12c1-...
- C Chemistry Lactic acid is a common by-product of cellular respiration and is often said to cause the burn associated with strenuous activity. a 25.0-ml sample of 0.100 m lactic acid (hc3h5o3,...
- C Chemistry The vapor pressure of water at 90°c is 0.692 atm. what is the vapor pressure (in atm) of a solution made by dissolving 1.17 mole(s) of csf(s) in 1.00 kg of water? assume that...
- C Chemistry The water level in a metric measuring cup is 0.75 l before the addition of 150. g of shortening. the water level after submerging the shortening is 0.92 l. determine the density...
- C Chemistry To what temperature does a 250.0-ml cylinder containing 0.1000 mol he need to be cooled in order for the pressure to be 253.25 kpa...
- C Chemistry A buffer solution that is 0.100 M in both HCOOH and HCOOK has a pH = 3.75. A student says that if a very small amount of 0.100 M HCl is added to the buffer, the pH will decrease...

Ответ:
The molecular formula for the given compound is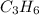
Explanation : Given,
Percentage of C = 85.7 %
Percentage of H = 14.3 %
Let the mass of compound be 100 g. So, percentages given are taken as mass.
Mass of C = 85.7 g
Mass of H = 14.3 g
To formulate the empirical formula, we need to follow some steps:
Step 1: Converting the given masses into moles.
Moles of Carbon =
Moles of Hydrogen =
Step 2: Calculating the mole ratio of the given elements.
For the mole ratio, we divide each value of the moles by the smallest number of moles calculated which is 7.14 moles.
For Carbon =
For Hydrogen =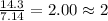
Step 3: Taking the mole ratio as their subscripts.
The ratio of C : H = 1 : 2
The empirical formula for the given compound is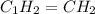
Mass of empirical formula =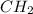 = 1(12) + 2(1) = 14 g/eq.
= 1(12) + 2(1) = 14 g/eq.
Now we have to determine the molar mass of compound.
As, 112 mL of volume contains 0.21 g of compound
So, 22400 mL of volume contains of compound
of compound
For determining the molecular formula, we need to determine the valency which is multiplied by each element to get the molecular formula.
The equation used to calculate the valency is :
We are given:
Mass of molecular formula = 42 g/mol
Mass of empirical formula = 14 g/mol
Putting values in above equation, we get:
Multiplying this valency by the subscript of every element of empirical formula, we get:
Thus, the molecular formula for the given compound is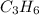
Ответ:
Out puts