vickie2370
27.08.2019 •
Chemistry
Argon (molecular weight 40 g/mole) is a monatomic compound. if liquid argon is confined to a container and held at a constant temperature of 80.5 k, what is the approximate vapor pressure of gaseous argon, assuming the liquid has no entropy and a binding energy of 0.1 ev? [note: at 1 atm, the boiling point is 87.3 k.]
Solved
Show answers
More tips
- S Society and Politics Why are thugs called gopniks ? A fascinating journey through Russian subculture...
- A Animals and plants Want a Perfect Lawn? Learn How to Plant Grass the Right Way...
- A Animals and plants How to Properly Care for a Pet Decorative Rabbit at Home?...
- C Computers and Internet How to Check the Speed of My Internet?...
- H Health and Medicine 10 Ways to Cleanse Your Colon and Improve Your Health...
- W Work and Career How to Write a Resume That Catches the Employer s Attention?...
- C Computers and Internet Е-head: How it Simplifies Life for Users?...
- F Family and Home How to Choose the Best Diapers for Your Baby?...
- F Family and Home Parquet or laminate, which is better?...
- L Leisure and Entertainment How to Properly Wind Fishing Line onto a Reel?...
Answers on questions: Chemistry
- C Chemistry An unknown gas effuses at a speed one quarter of that of helium. what is the molar mass of the unknown gas? it is either sulfur di oxide or sulfur tri oxide. which gas is it....
- C Chemistry You don’t have to answer everything, whatever you know could be really 1) what is the molarity of a 5 l solution containing 450g of nacl made from 4.5kg of water? 2) there is 6.00...
- C Chemistry Which statement goes against the kinetic theory of gases? a.the size of gas molecules is negligible compared to the volume of the container in which they are kept. b. gas molecules...
- C Chemistry Which of the following statements is true about hydropower? It releases carbon dioxide. It can produce harmful chemicals. It can affect fish and other marine life. It uses gas trapped...
- C Chemistry Diamond has a density of 3.52 g/ml. what is the volume in cubic centimeters of a diamond with a mass of 15.1 g? 4.3 cm3 4.29 cm3...
- C Chemistry Convert -12.00 degrees celsius to the unit kelvin. 296.15 k 261.15 k -285.15 k -162.85 k...
- C Chemistry How does the jet stream move storms across north america?...
- E English Choose the simple sentence...
- M Mathematics The incomplete table and histogram give information about the waiting time, in minutes, experienced by patients at a dental surgery. A)use the histogram to complete the table.B)Use...
- E English Dr. Rank suggests Nora should go to the next masquerade dressed as Charmed Life, and that she should dress just as she looks every day. What is the implication about Nora s daily life?...

Ответ:
Explanation:
As the given data is as follows.
and,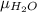 =
= 
T = 80.5 K
According to the given condition,
p = nkT =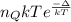
Therefore, putting the given values into the above equation as follows.
=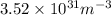
Therefore, the required pressure is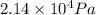 .
.
Ответ:
Respuesta
es la C. silicio
Explanation:
No existe una definición estandarizada de elemento metaloide ni un consenso completo sobre los elementos que son metaloides.
En la clasificación de metaloides se suelen incluir a:
Boro (B)
Silicio (Si)
Germanio (Ge)
Arsénico (As)
Antimonio (Sb)
Telurio (Te)