officialrogerfp3gf2s
12.09.2019 •
Chemistry
As a technician in a large pharmaceutical research firm, you need to produce 350. ml of 1.00 m potassium phosphate buffer solution of ph = 7.07. the pka of h2po4− is 7.21. you have the following supplies: 2.00 l of 1.00 m kh2po4 stock solution, 1.50 l of 1.00 m k2hpo4 stock solution, and a carboy of pure distilled h2o. how much 1.00 m kh2po4 will you need to make this solution?
Solved
Show answers
More tips
- S Style and Beauty What Peeling is Suitable for Sensitive Skin?...
- A Auto and Moto What Is the Cost of Customs Clearance for a Car in Russia?...
- L Leisure and Entertainment Should You Buy a Ceramic Knife?...
- C Computers and Internet How to easily and quickly disable Firebug in Gmail and Google Docs...
- G Goods and services How to sew a ribbon: Tips for beginners...
- F Food and Cooking How to Make Mayonnaise at Home? Secrets of Homemade Mayonnaise...
- C Computers and Internet Which Phone is Best for Internet Surfing?...
- F Food and Cooking Everything You Need to Know About Pasta...
- C Computers and Internet How to Choose a Monitor?...
- H Horoscopes, Magic, Divination Where Did Tarot Cards Come From?...
Answers on questions: Chemistry
- C Chemistry Clasifica según su carácter metálico los siguientes elementos mercurio azufre calcio estaño cerio plata fósforo y silicio...
- C Chemistry Explain the importance of groundwater to communities....
- C Chemistry Duck bc so oh go tv nbc do pc cut and kb chu gc...
- C Chemistry Los metales que usos an tenido atravez de la historia...
- C Chemistry 17.For each of the following bonds, draw a figure indicating the direction of the bond dipole, including which end of the bond is positive and which is negative. a. S-P b. S-0 C....
- C Chemistry 2. How would type of substance affect the rate of reaction?...
- C Chemistry Chemical name for Fe2O3?...
- M Mathematics How can i solve x^3+7x=8x^2...
- M Mathematics What percent of 300 is 90. Help please...
- M Mathematics 4a²+4a x a²-5aa²-25 8a...

Ответ:
You need to add 203 mL of 1,00M KH₂PO₄ and 147 mL of 1,00M K₂HPO₄.
Explanation:
It is possible to use Henderson–Hasselbalch equation to estimate pH in a buffer solution:
pH = pka + log₁₀
Where A⁻ is conjugate base and HA is conjugate acid
The equilibrium of phosphate buffer is:
H₂PO₄⁻ ⇄ HPO4²⁻ + H⁺ Kₐ₂ = 6,20x10⁻⁸; pka=7,21
Thus, Henderson–Hasselbalch equation for 7,07 phosphate buffer is:
7,07 = 7,21 + log₁₀![\frac{[HPO4^{2-}] }{[H2PO4^{-}]}](/tpl/images/0229/1588/94f69.png)
0,7244 =![\frac{[HPO4^{2-}] }{[H2PO4^{-}]}](/tpl/images/0229/1588/94f69.png) (1)
(1)
As the buffer concentration must be 1,00 M:
1,00 = [H₂PO₄⁻] + [HPO4²] (2)
Replacing (2) in (1):
[H₂PO₄⁻] = 0,5799 M
Thus:
[HPO4²] = 0,4201 M
To obtain these concentrations you need to add:
0,5799 M × 0,350 L ×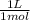 = 0,203 L ≡ 203 mL of 1,00M KH₂PO₄
= 0,203 L ≡ 203 mL of 1,00M KH₂PO₄
And:
0,4201 M × 0,350 L ×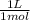 = 0,203 L ≡ 147 mL of 1,00M K₂HPO₄
= 0,203 L ≡ 147 mL of 1,00M K₂HPO₄
I hope it helps!
Ответ:
C. Physical Change.