lorenzomendi1011
02.03.2020 •
Chemistry
As part of Lab 4 you will make and standardize a solution of NaOH(aq). Suppose in the lab you measure the solid NaOH and dissolve it into 100.0 mL of water. You then measure 0.2000 g of KHP (204.22 g/mol) and place it in a clean, dry 100-mL beaker, and then dissolve the KHP in about 25 mL of water and add a couple of drops of phenolphthalein indicator. You titrate this with your NaOH(aq) solution and find that the titration requires 9.78 mL of NaOH(aq). Part 1: How many moles of KHP are in your sample
Solved
Show answers
More tips
- S Style and Beauty Choosing a Hair Straightener: Specific Criteria to Consider...
- F Family and Home How to Remove Tar Stains: Tips and Recommendations from Experts...
- F Family and Home How to Remove Fading from Clothes: Tips and Tricks...
- S Sport How to Do a Jumping Split...
- H Health and Medicine How Did Inna Lose Weight on Dom 2?...
- F Family and Home How to Properly Fold Napkins in a Napkin Holder?...
- F Food and Cooking How to Set Up Ventrilo - The Ultimate Guide...
- S Science and Technology How to Make a Homemade Smoker: The Ultimate Guide...
- A Auto and Moto Battle for the Relocation of The Cherkizovsky Market: Who Won?...
- C Computers and Internet How Do You Refill Cartridges?...
Answers on questions: Chemistry
- C Chemistry Suzy steps on the scale and measures her weight to be 125 lbs. How much does she weigh in kg? Select one: O a 125 kg O b. 0.0176 kg O c.56.8 kg O d. 275 kg...
- C Chemistry What do u know about a gas s molecules...
- C Chemistry Which of the following solutions would not be expected to exist? Group of answer choices NaCl in CCl4 (carbon tetrachloride, a nonpolar solvent) Pentane, C5H12, in CCl4 Methyl alcohol,...
- C Chemistry A sample of lithium is held at 1000 K. What phase of matter would the sample be in?...
- M Mathematics Landon and Ella are dinner at Billy’s barbeque. The cost was $25.50. They left the waiter a 20% tip how munch Money did Landon and Ella leave for a tip...
- C Chemistry What is the difference between active transport? Which one requires energy?...
- E English Whats the weird food combination that you actually like...
- M Mathematics Daisy works at an ice-cream parlor. She is paid $10 per hour for the first 8 hours in a day. For every extra hour she works, she is paid 1.5 times her hourly wage. If Daisy works...
- G Geography Many people believe that a large earthquake, often referred to as the Big One; will hit California within the next 100 years. Due to a lack of understanding about the earth s plates,...
- H History This is a map of Asia...

Ответ:
The moles of KHP in the sample is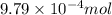
Explanation:
To calculate the number of moles, we use the equation:
Given mass of KHP = 0.2000 g
Molar mass of KHP = 204.22 g/mol
Putting values in above equation, we get:
Hence, the moles of KHP in the sample is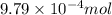
Ответ:
Adjusting the Flame. Use the needle valve to control the flame's size. The needle valve on the bottom of the Bunsen burner adjusts the gas flow rate, which determines the height of the flame. More gas will create a larger flame, and less gas gives you a smaller flame.
Explanation:
Hope it is helpful