Aspirin (c9h8o4, molecular mass 180.2 g/mol) is synthesized by the reaction of salicylic acid (c7h6o3, molecular mass 138.1 g/mol) with acetic anhydride (c4h6o3, molecular mass 102.1 g/mol) according to the reaction 2 c7h6o3 (s) + c4h6o3(l) → 2c9h8o4(s)+ h2o(l) 2.0g of salicylic acid and 5.4 g of acetic anhydride are mixed and the reaction is allowed to go to completion. do each of the following: a. identify the limiting reagent. b. state how many grams of the excess reagent remain. c. state how many grams of product are produced.
Solved
Show answers
More tips
- F Food and Cooking What is the Right Thickness for a Perfect Cream Puff?...
- F Food and Cooking What can and cannot be eaten during Lent?...
- F Food and Cooking How to Find Your Zip Code?...
- W Work and Career Can Skill Alone Make You a Professional?...
- C Computers and Internet How to Top Up Your Skype Account Without Losing Money?...
- P Philosophy Unidentified Flying Object - What is the Nature of this Phenomenon?...
- F Family and Home Protect Your Home or Apartment from Pesky Ants...
- O Other What is a Disk Emulsifier and How Does it Work?...
- F Family and Home What does a newborn need?...
Answers on questions: Chemistry
- C Chemistry Veja a entalpia-padrão de formação, em KJ.mol-1 e a 25°C, de algumas substâncias: CH4(g) -74,8; CHCl3(l) - 134,5; HCl(g) - 92,3 Se realizarmos a reação de cloração do metano, qual...
- C Chemistry What is an application of colligative properties in real life?...
- C Chemistry Practice Problem 15.59Get help answering Molecular Drawing questionsGet help answering Molecular Drawing questions.Draw the structure of a compound with the molecular formula C9H10O2...
- C Chemistry A helium nucleus with two protons and two neutrons is called a(n) A. alpha particle B. beta particle C. electroscope D. gamma ray Clear selection...
- C Chemistry What mass of carbon dioxide is produced by the reaction of 8.8 g of octane ?...
- C Chemistry At STP How many mole are .00320 mol co2...
- C Chemistry Show your work including units in your answer. Calculate the molar mass of P3(SO2),...
- M Mathematics Rationalize the denominator of $\frac{\sqrt{5}+\sqrt{2}}{\sqrt{5}-\sqrt{2}}$. The answer can be written as $\frac{A+B\sqrt{C}}{D}$, where $A$, $B$, $C$, and $D$ are integers, $D$...
- B Biology 3. Complete the table by defining reduce, reusable and recyclable in your own words .REDUCERECYCLABLEREUSABLE...
- C Chemistry Based on the particular theory, explain why smoke flow easily in all directions....

Ответ:
A. The Salicylic acid is the limiting reagent.
is the limiting reagent.
B. 4.66 g of the acetic anhydride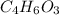 remain.
remain.
C. 2.6 g of are produced.
are produced.
Explanation:
A. First write the balanced chemical equation fior the asprin synthesis:
Then determine the limiting reagent.
To determine the limiting reagent first divide the mass of each compound between its molar mass, then divide this quantity by the number of moles given by the reaction and the smallest number will be the limiting reagent.
- For the Salicylic acid :
:
- For the Acetic anhydride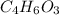 :
:
The smallest number is for the salicylic acid therefore it is the limiting reagent.
B. Calculate how many grams of the excess reagent remain.
- First calculate how many grams of acetic anhydride reacts:
- Then subtract the quantity of acetic anhydride that reacts from the quantity that are mixed:
C. Calculate how many grams of product are produced:
Ответ:
by multiply
Explanation: