At elevated temperatures, dinitrogen pentoxide decomposes to nitrogen dioxide and oxygen: 2N2O5(g)→4NO2(g)+O2(g) Part A When the rate of formation of O2 is 8.5×10−3 M/s, the rate of decomposition of N2O5 is M/s.
Solved
Show answers
More tips
- A Animals and plants What Do Terriers Eat?...
- F Food and Cooking Discover the Benefits and Properties of Dates...
- C Computers and Internet Dynamically Assigned IP Address: What Is It and How Does It Work?...
- S Style and Beauty How to Get Rid of Acne: Scientifically Proven Methods...
- H Health and Medicine Simple Ways to Lower Cholesterol in the Blood: Tips and Tricks...
- O Other How to Choose the Best Answer to Your Question on The Grand Question ?...
- L Leisure and Entertainment History of International Women s Day: When Did the Celebration of March 8th Begin?...
- S Style and Beauty Intimate Haircut: The Reasons, Popularity, and Risks...
- A Art and Culture When Will Eurovision 2011 Take Place?...
- S Style and Beauty How to Choose the Perfect Hair Straightener?...
Answers on questions: Chemistry
- C Chemistry Which statement comparing chemical and nuclear properties of isotopes is correct? isotopes have similar chemical and nuclear properties. isotopes have different chemical and nuclear...
- C Chemistry Which of the following statements is correct? 1. the laboratory experiment allows greater control of extraneous variables than the field experiment. 2. the independent variable...
- C Chemistry 35 POINTS Y ALL What is the difference in petroleum and natural gas? thanks, -throckmorton...
- C Chemistry What does the name 2–butene tell you about this hydrocarbon s molecular structure?...
- H History ONE MULTIPLE CHOICE QUESTION HELPPPPPPP...
- H History geographically describe the locations of us territorial acquisitions, what might have contributed to these patterns...
- H History I’ll mark brainliest plzzz help A dynasty is best defined as a A. book about royalty. B. decade of royal rule. C. family that passes on its right to rule from one generation to...
- C Chemistry Consider the following equilibrium: 2SO^2(g) + O2(9) = 2 SO3^(g) 1. What is equal at equilibrium?2. What would happen to the forward rate if some 0were removed from this equilibrium?3....
- M Mathematics \dfrac{-2}{3} \div 2\dfrac{1}{4} = 3 −2 ÷2 4 1...
- M Mathematics Carson has a beach ball with a diameter of 50 cm what is the approximate volume of the beach ball? use 3.14 as an approximation for Pi...

Ответ:
Rate of disappearance of N₂O₅ = 17 x 10⁻³ M/sec
Explanation:
Rate of reaction =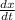
Where
dx = Change of concentration of reactants or products
dt = Change in time
According to chemical reaction
2 N₂O₅(g) → 4 NO₂(g)+O₂(g)
Rate of disappearance of N₂O₅ = Rate of appearance of O₂
Ответ:
1. Mixtures
2. Different
3. Manipulations
4. Magnet
5. Filtration
6. Liquid
7. Evaporation