At equilibrium, a 1.0 L reaction vessel contains 2.3 mol of Mg(OH)₂ and 0.170 mol of OH⁻. What is the equilibrium concentration of Mg²⁺ based on the reaction:
Mg(OH)₂ (s) ⇌ Mg²⁺ (aq) + 2 OH⁻ (aq) Kc = 1.80 x 10⁻¹¹
Solved
Show answers
More tips
- F Food and Cooking Аэрогриль: Все, что нужно знать для подготовки здоровой и вкусной пищи...
- G Goods and services How to choose a washing machine?...
- F Family and Home Is it Worth Knowing the Gender of Your Child Before Birth?...
- H Health and Medicine Mercury Thermometer Danger: What to do when a thermometer breaks?...
- F Food and Cooking How to cook crayfish? Everything you need to know...
- G Goods and services LED-подсветка в LCD-телевизорах: 5 причин, почему она лучше других технологий...
- P Photography and Videography Understanding HDR: How It Works and Why You Need It...
- G Goods and services Which TV is better - LCD or Plasma?...
- S Sport How to Learn to Pull Up on Monkey Bars?...
- L Leisure and Entertainment Scrapbooking: What is it and Why is it Becoming More Popular?...
Answers on questions: Chemistry
- C Chemistry To completely neutralise 200cm3 of 0.5mol/dm3 sodium hydroxide (NaOH), a student adds 100cm3 of 0.5mol/dm3 sulfuric acid (H2SO4). The temperature of the solution...
- C Chemistry Propose a mechanism for the following reaction...
- C Chemistry 24.50 g of Na react with H20 to make sodium hydroxide and H2 gas. How much sodium hydroxide is made if there is an excess of water?...
- C Chemistry The ocean does NOT influencepur weather. true or false...
- C Chemistry A 3.6 g sample of iron (III) oxide reacts with sufficient aluminum to be entirely used up. How much iron is produced?...
- C Chemistry Which bond type accounts for the reaction between boron triflouride and ammonia...
- C Chemistry A piece of metallic iron (10 moles) was dissolved in concentrated hydrochloric acid the reaction formed hydrogen gas and iron chloride how many grams of HCI were...
- C Chemistry How many elements are present in each of the following HF and. Hf ii) Co and CO iii) Si and SiO2 iv) PoCl2 and POCC3...
- C Chemistry A 0.954 g sample of an unknown acid H2A reacts completely with 6.80 mL of a 10 % NaOH (% m/m) solution that has a density of 1.08 g mL.Calculate the molar mass of...
- C Chemistry The two must abundant elements in seawater are and ....

Ответ:
At equilibrium, a 1.0 L reaction vessel contains 2.3 mol of Mg(OH)₂ and 0.170 mol of OH⁻. The equilibrium concentration of Mg²⁺ based on the reaction is 0.622 × 10⁻⁹.
The equilibrium concentration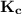 relates to the summation of the initial concentration and the change gotten from its stoichiometric reaction.
relates to the summation of the initial concentration and the change gotten from its stoichiometric reaction.
From the reaction given, the equilibrium concentration can be represented as:
![\mathbf{K_c = \dfrac{[Mg^{2+}_{(aq)}] [OH^-]^2}{[Mg(OH)_2_{(s)}]} }](/tpl/images/0611/5877/2058a.png)
The concentration of the solid substance of [Mg(OH)₂] = 1.0 MConcentration of [OH]⁻ = no of moles/volume= 0.170 M/1.0 L = 0.170 M∴
Learn more about the equilibrium constant here:
link
Ответ:
The value of X for this chemical reaction is 1.65 x 10⁻⁴
Explanation:
Mg (OH)₂(s) <==> Mg²⁺+(aq) + 2OH₋₁(aq)
Kc= 1.80x10⁻¹¹
KC = [Mg²⁺][OH-]²
Let us assume
Put X = [Mg²⁺] and 2x = [OH-]
1.80x10-11 = (X) (2X)²
1.80x10-11 = 4X³
X³ = 4.5x10⁻¹²
On simplifying the equation we get,
X = 1.65 x 10⁻⁴
The value of X for this chemical reaction is 1.65 x 10⁻⁴
Ответ:
Hope this would help~