Balance the following oxidation-reduction reactions, which occur in acidic solution, using the half-reaction method.
(use the lowest possible coefficients. include states-of-matter under the given conditions in your answer.)
o2(g) + pb(s) → h2o(l) + pb2+(aq) (b) no3−(aq) + sn(s) → no(g) + sn2+(aq) (c) cl2(g) + cr3+(aq) → cl −(aq) + cr2o72−(aq) (d) f2(g) + mn2+(aq) → f −(aq) + mno4−(aq)
Solved
Show answers
More tips
- A Animals and plants Want a Perfect Lawn? Learn How to Plant Grass the Right Way...
- A Animals and plants How to Properly Care for a Pet Decorative Rabbit at Home?...
- C Computers and Internet How to Check the Speed of My Internet?...
- H Health and Medicine 10 Ways to Cleanse Your Colon and Improve Your Health...
- W Work and Career How to Write a Resume That Catches the Employer s Attention?...
- C Computers and Internet Е-head: How it Simplifies Life for Users?...
- F Family and Home How to Choose the Best Diapers for Your Baby?...
- F Family and Home Parquet or laminate, which is better?...
- L Leisure and Entertainment How to Properly Wind Fishing Line onto a Reel?...
- L Leisure and Entertainment How to Make a Paper Boat in Simple Steps...
Answers on questions: Chemistry
- C Chemistry What is the molarity of a 0.30 liter solution containing 0.50 moles of NaCl?...
- C Chemistry How much heat is needed to take 15.0 grams of ICE at 0 C to water at 50.5 C?...
- C Chemistry Acid rain water breaks down Rocks by weathering...
- C Chemistry 1. A football player throws a football 30 meters/second2 using 600 Newtons of force What was the mass of the football?...
- C Chemistry Base your answer to the question on the information below. Some dry chemicals can be used to put out forest fires. One of these chemicals is NaHCO3. When NaHCO3(s) is...
- C Chemistry V1 =744 mL, P1 =785 mm of Hg, V2 = 256 mL, P2 = ?...
- C Chemistry Chemistry common assessment 3rd 9 weeks...
- C Computers and Technology Which best describes how computer simulations are used in science? -a)computer simulations scientists see very large objects. -b)computer simulations scientists see...
- H History Which type of clothing emphasized femininity? a. military-style dresses b. jersey dresses c. dashiki dresses d. nipped-in waist dresses e. tennis dresses...
- C Chemistry Two gas jars are connected to each other, but they are separated by a closed valve. one gas jar contains oxygen, and the other is empty. what will happen when the valve...

Ответ:
Answer : The balanced chemical equation in a acidic solution are,
(a)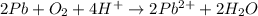
(b)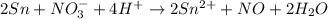
(c)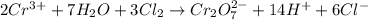
(d)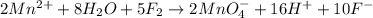
Explanation :
Redox reaction or Oxidation-reduction reaction : It is defined as the reaction in which the oxidation and reduction reaction takes place simultaneously.
Oxidation reaction : It is defined as the reaction in which a substance looses its electrons. In this, oxidation state of an element increases. Or we can say that in oxidation, the loss of electrons takes place.
Reduction reaction : It is defined as the reaction in which a substance gains electrons. In this, oxidation state of an element decreases. Or we can say that in reduction, the gain of electrons takes place.
(a) The given chemical reaction is,
The oxidation-reduction half reaction will be :
Oxidation :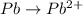
Reduction :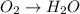
Now balance oxygen atom on both side.Oxidation :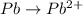
Reduction :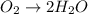
Now balance hydrogen atom on both side.Oxidation :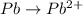
Reduction :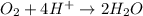
Now balance the charge.Oxidation :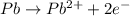
Reduction :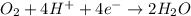
In order to balance the electrons, we multiply the oxidation reaction by 2 and then added both equation, we get the balanced redox reaction.
Oxidation :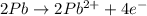
Reduction :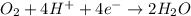
The balanced chemical equation will be,
(b) The given chemical reaction is,
The oxidation-reduction half reaction will be :
Oxidation :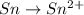
Reduction :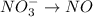
Now balance oxygen atom on both side.Oxidation :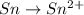
Reduction :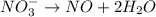
Now balance hydrogen atom on both side.Oxidation :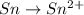
Reduction :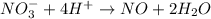
Now balance the charge.Oxidation :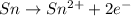
Reduction :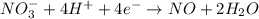
In order to balance the electrons, we multiply the oxidation reaction by 2 and then added both equation, we get the balanced redox reaction.
Oxidation :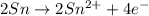
Reduction :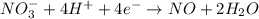
The balanced chemical equation will be,
(c) The given chemical reaction is,
The oxidation-reduction half reaction will be :
Oxidation :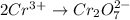
Reduction :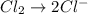
Now balance oxygen atom on both side.Oxidation :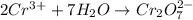
Reduction :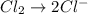
Now balance hydrogen atom on both side.Oxidation :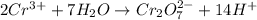
Reduction :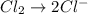
Now balance the charge.Oxidation :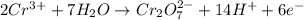
Reduction :
In order to balance the electrons, we multiply the reduction reaction by 3 and then added both equation, we get the balanced redox reaction.
Oxidation :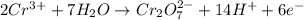
Reduction :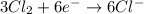
The balanced chemical equation will be,
(d) The given chemical reaction is,
The oxidation-reduction half reaction will be :
Oxidation :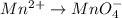
Reduction :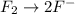
Now balance oxygen atom on both side.Oxidation :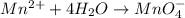
Reduction :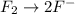
Now balance hydrogen atom on both side.Oxidation :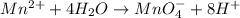
Reduction :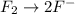
Now balance the charge.Oxidation :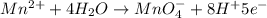
Reduction :
In order to balance the electrons, we multiply the oxidation reaction by 2 and reduction reaction by 5 and then added both equation, we get the balanced redox reaction.
Oxidation :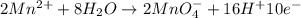
Reduction :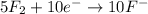
The balanced chemical equation will be,
Ответ:
don't get people on the internet to do your test for you. once day you will greatly benefit from this warning. Kind regards
Explanation: