sedilei1515
08.07.2019 •
Chemistry
Be sure to answer all parts. propane (c3h8) is a minor component of natural gas and is used in domestic cooking and heating. (a) balance the following equation representing the combustion of propane in air. include states of matter in your answer. c3h8(g) + o2(g) → co2(g) + h2o(g) (b) how many grams of carbon dioxide can be produced by burning 8.11 moles of propane? assume that oxygen is the excess reactant in this reaction. × 10 g enter your answer in scientific notation.
Solved
Show answers
More tips
- C Computers and Internet Are there special gaming mice?...
- L Leisure and Entertainment When will Maslenitsa start?...
- F Food and Cooking Discovering the Mysterious Fruit of Feijoa...
- B Business and Finance How to Open an Online Store? A Detailed Guide for Beginners...
- W Work and Career How to Write a Resume That Catches the Employer s Attention?...
- C Computers and Internet Е-head: How it Simplifies Life for Users?...
- F Family and Home How to Choose the Best Diapers for Your Baby?...
- F Family and Home Parquet or laminate, which is better?...
- L Leisure and Entertainment How to Properly Wind Fishing Line onto a Reel?...
- L Leisure and Entertainment How to Make a Paper Boat in Simple Steps...
Answers on questions: Chemistry
- M Mathematics Question 1 (1 point) How would the parent function, f(x)=2x , be transformed if the function was changed tof(x)=−2x ? Question 1 options: The new graph would reflect across...
- S Social Studies Eric is a high school student, and he does not have any paid work experience yet. what information could he include in the work experience section of his résumé? a. school...

Ответ:
For a: The balanced chemical equation is given below.
For b: The mass of carbon dioxide produced will be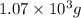
Explanation:
For a:Every balanced chemical equation follows law of conservation of mass.
This law states that mass can neither be created nor can be destroyed but it can only be transformed from one form to another form.
This law also states that the total number of individual atoms on the reactant side must be equal to the total number of individual atoms on the product side.
For the given reaction, the balance chemical equation follows:
All the substances are present in gaseous state.
For b:By Stoichiometry of the reaction:
1 mole of propane gas produces 3 moles of carbon dioxide gas.
So, 8.11 moles of propane gas will produce =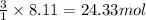 of carbon dioxide gas.
of carbon dioxide gas.
Now, calculating the mass of carbon dioxide using equation:
Molar mass of carbon dioxide = 44 g/mol
Moles of carbon dioxide = 24.33 mol
Putting values in above equation, we get:
Hence, the amount of produced in the given reaction and expressed in scientific notation is
produced in the given reaction and expressed in scientific notation is 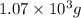
Ответ: