Calcium chloride, cacl2, is commonly used as an electrolyte in sports drinks and other beverages, including bottled water. a solution is made by adding 6.50 g of cacl2 to 60.0 ml of water at 25∘c. the density of the solvent at that temperature is 0.997 g/ml. calculate the mole percent of cacl2 in the solution.
Solved
Show answers
More tips
- A Animals and plants Money Tree Care Secrets: How to Keep Your Plant Thriving...
- F Food and Cooking Delight for Gourmets: How to Prepare Liver Pate...
- S Style and Beauty How to braid friendship bracelets?...
- H Health and Medicine Mercury Thermometer Danger: What to do when a thermometer breaks?...
- F Food and Cooking Which Calamari Salad is the Most Delicious?...
- S Society and Politics 10 Tips for Boosting Your Self-Esteem...
- F Food and Cooking The Most Delicious and Simple Fish in Batter Recipe...
- H Health and Medicine What is Autism? Understanding the Basics of This Neurodevelopmental Disorder...
- P Philosophy How to Develop Extrasensory Abilities?...
- S Style and Beauty Don t Sacrifice Your Brows: How to Properly Pluck Stubborn Hairs...
Answers on questions: Chemistry
- C Chemistry What’s is line A and what is Line B? Please help...
- C Chemistry Balance the following equation then determine the mole ratio for AlBr, and Al AlBr3+ K---→KBr+Αl please show how you got it...
- C Chemistry Edward is gathering physical evidence at the scene of a crime. He finds a fingerprint pressed into the wax of a candle. What type of fingerprint is this?...
- C Chemistry Name the five structures that a write may use to construct his/ her writing....
- C Chemistry How is drip irrigation better than canal irrigation?Explain as well...
- C Chemistry All of the orbitals in a given subshell have the same value of the quantum number...
- C Chemistry How many grams of sodium carbonate, Na2CO3, are needed to add to 0.125 kg of water to make a 0.200 m (molal) solution...
- C Chemistry How would you figure the number of protons or electrons in an atom...
- M Mathematics A certain human red blood cell has a diameter of 0.000007 meters. Which expression represents this diameter, in meters, in scientific notation? Group of answer choices 7×10−6 7×105...
- M Mathematics Cara computes the mean and variance for the set 87, 46, 90, 78, and 89. She finds the mean to be 78. Her steps for finding the variance are shown below. o2 - 187–7852 + (45–78)? +(90...

Ответ:
Mole percent of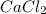 in solution is 1.71%
in solution is 1.71%
Explanation:
Number of moles of a compound is the ratio of mass to molar mass of the compound.
Molar mass of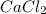 = 110.98 g/mol
= 110.98 g/mol
Molar mass of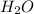 = 18.02 g/mol
= 18.02 g/mol
Density is the ratio of mass to volume
So, mass of 60.0 mL of water =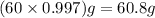
Hence, 6.50 g of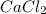 =
=  of
of 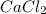 = 0.0586 moles of
= 0.0586 moles of 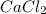
60.8 g of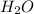 =
= 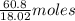 of
of 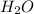 = 3.37 moles of
= 3.37 moles of 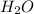
So, mole percent of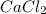 in solution = \frac{n_{CaCl_{2}}}{n_{total}}\times 100% =
in solution = \frac{n_{CaCl_{2}}}{n_{total}}\times 100% = 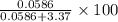 % = 1.71%
% = 1.71%
Ответ:
second 1
Step-by-step explanation: