Calculate the value of the free energy change, ΔG, for the reaction below at 750.0ºC when the pressures of POCl3 (g) = 10.00 atm, PCl3 (g) = 0.0150 atm, and O2 (g) = 0.0100 atm.
2 POCl3 (g) →2 PCl3 (g) + O2 (g)
ΔGº = 489.75 kJ
ΔHº = 542.8 kJ
ΔSº = 177.93 J/K
Solved
Show answers
More tips
- P Philosophy What is Something for you?...
- S Style and Beauty Intimate Haircut: The Reasons, Popularity, and Risks...
- A Art and Culture When Will Eurovision 2011 Take Place?...
- S Style and Beauty How to Choose the Perfect Hair Straightener?...
- F Family and Home Why Having Pets at Home is Good for Your Health...
- H Health and Medicine How to perform artificial respiration?...
- H Health and Medicine 10 Tips for Avoiding Vitamin Deficiency...
- F Food and Cooking How to Properly Cook Buckwheat?...
- F Food and Cooking How Many Grams Are In a Tablespoon?...
- L Leisure and Entertainment Carving: History and Techniques for Creating Vegetable and Fruit Decorations...
Answers on questions: Chemistry
- C Chemistry 80% of all earthquakes have occurred here and over 75% of the world s active and dormant volcanoes can be found around the Pacific Ocean Basin. This region is called:...
- C Chemistry state why oils from plant source remain liquid at 25°C but that of animal source remain solid at the same temperature...
- C Chemistry Which process converts mass into energy? fusion of hydrogen atoms ionization of cesium atoms filtration of a mixture distillation of ethanol?...
- C Chemistry 1. when magma solidifies underground, the resulting landform is classified as 2. lava cooling on the surface of the earth forms features 3. a very large mineral is more likely to be...
- C Chemistry Which process is a chemical change? (1) melting of ice (2) boiling of water (3) subliming of ice (4) decomposing of water...
- C Chemistry If one body is positively charged and another body is negatively charged, free electrons tend to a. move from the negatively charged body to the positively charged body. b. remain...
- C Chemistry In a solution of brine (salt water), what is the salt? a solute a solvent a solution a reactant a product...
- B Business On December 31, year 3, Byte Co. had capitalized software costs of $600,000 with an economic life of four years. Sales for year 4 were 10% of expected total sales of the software....
- M Mathematics Ivy is running 3 miles per hour She currently has run none Whichstatement best describes Ivy s situation?Ivy s situation is non-proportional because she will not be running milesat...
- C Chemistry What particle is the only one that determine the type of element you have...

Ответ:
the value of the free energy change ΔG = 339.975 kJ
Explanation:
The equation for the reaction is given as :
Equilibrium Constant K:
ΔGº = 489.75 kJ = 489750 J
T (temperature) = 750.0ºC = ( 750.0 + 273 )K
T (temperature) = 1023 K
R( rate constant) =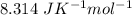
Using the equation :
ΔG = ΔG° + RT ㏑ K
ΔG = (489750) + ( 8.314)(1023)㏑ ( 2.25 × 10⁻⁸)
ΔG = 489750 + 8505.22 × (-17.6098)
ΔG = 339974.78 J
ΔG = 339.975 kJ
Thus, the value of the free energy change ΔG = 339.975 kJ
Ответ:
i would help but its in spanish sorry
Explanation: