ralewis2931
11.07.2019 •
Chemistry
Calculate the volumetric size of a water molecule in water vapor at normal conditions, assuming 1 mole of the vapor occupies 22.4 l, as if the vapor were an ideal gas. give answer in angstroms, two significant digits. do not write down units in your answer.calculate the volumetric size of a water molecule in water vapor at normal conditions, assuming 1 mole of the vapor occupies 22.4 l, as if the vapor were an ideal gas. give answer in angstroms, two significant digits. do not write down units in your answer.
Solved
Show answers
More tips
- H Health and Medicine Coughing: Causes, Types, and Treatment Methods...
- H Health and Medicine How to Treat the Flu: A Comprehensive Guide...
- O Other What is a Disk Emulsifier and How Does it Work?...
- H Health and Medicine How to Calm Your Nerves? Expert Tips That Actually Work...
- A Animals and plants 5 Tips for Taking Care of Yews to Keep Them Green and Beautiful...
- S Sport How to wrap boxing hand wraps? Everything you need to know!...
- F Food and Cooking 10 Reasons Why You Should Avoid Giving Re-Gifts: An Informative Guide...
- F Family and Home Tender Care for Your Parquet: Is it Possible to Clean Parquet?...
- S Style and Beauty How Are Eyelash Extensions Applied? All Your Questions Answered...
- F Food and Cooking 10 Tips for Proper Sushi Consumption...
Answers on questions: Chemistry
- S Spanish InstructionsListen to the conversation between Camilo and Sofía and complete Sofía’s schedule....
- P Physics Answer! will mark brainliest! which of the following is most likely to form when hot magma rises up as tectonic plates move apart below the ocean? mid-ocean ridge...
- E English Write a well-developed paragraph explaining the positive and significant impact that the person you researched had on the world. Read through the attached rubric...
- M Mathematics there are 18 cups on the shelves in Mrs. Lee s kitchen. If she takes out 8 cups to serve tea, how many cups will be left?...

Ответ:
Let volumetric size of a water molecule = v
Since, 1 mole of water consists of molecules of water.
molecules of water.
Thus, total volume of 1 mole of water =
Substitute the value of total volume of 1 mole of water i.e. 22.4 L in above formula.
Convert the unit litre to angstrom
=
=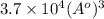
Therefore, the volumetric size of the water molecule is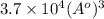
Ответ:
2.29x10⁻¹² is Ksp of the salt
Explanation:
The Ksp of the metal hydroxide is:
M(OH)₂(s) ⇄ M²⁺ + 2OH⁻
Ksp = [M²⁺] [OH⁻]²
As you can see in the reaction, 2 moles of OH⁻ are produced per mole of M²⁺. It is possible to find [OH⁻] with pH, thus:
pOH = 14- pH
pOH = 14 - 10.22
pOH = 3.78
pOH = -log[OH⁻]
1.66x10⁻⁴ = [OH⁻]
And [M²⁺] is the half of [OH⁻], [M²⁺] = 8.30x10⁻⁵
Replacing in Ksp formula:
Ksp = [8.30x10⁻⁵] [1.66x10⁻⁴]²
Ksp = 2.29x10⁻¹² is Ksp of the salt