lukerothbacher
13.03.2021 •
Chemistry
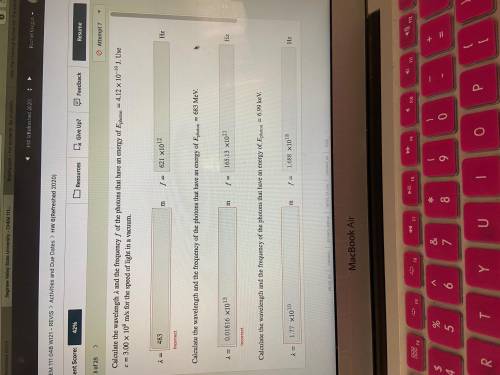
Calculate the wavelength and the frequency of the photons that have an energy of photon=4.12×10−19 J. Use =3.00×108 m/s for the speed of light in a vacuum.
Calculate the wavelength and the frequency of the photons that have an energy of photon=683 MeV.
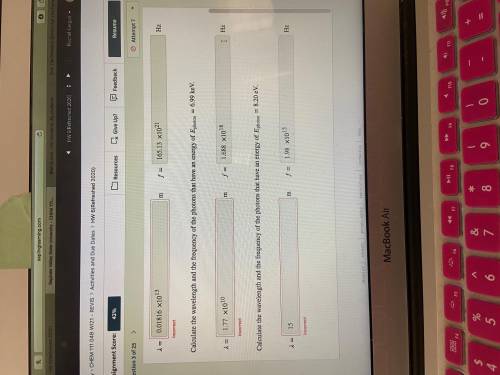
Calculate the wavelength and the frequency of the photons that have an energy of photon=6.99 keV.
Calculate the wavelength and the frequency of the photons that have an energy of photon=8.20 eV.
Solved
Show answers
More tips
- S Style and Beauty How to Get Rid of a Double Chin?...
- F Food and Cooking How to Cook Julienne? Recipes and Tips...
- D Dating, Love, Relationships 10 Useful Tips on How to Survive a Breakup?...
- F Food and Cooking Apple Cider Vinegar: The Ultimate Health and Beauty Solution...
- C Computers and Internet Е-head: How it Simplifies Life for Users?...
- F Family and Home How to Choose the Best Diapers for Your Baby?...
- F Family and Home Parquet or laminate, which is better?...
- L Leisure and Entertainment How to Properly Wind Fishing Line onto a Reel?...
- L Leisure and Entertainment How to Make a Paper Boat in Simple Steps...
- T Travel and tourism Maldives Adventures: What is the Best Season to Visit the Luxurious Beaches?...
Answers on questions: Chemistry
- M Mathematics Now let’s be as general as possible by not identifying which base we are in. just call the base x. consider the expression 1∙x³+2∙x²+7∙x+3∙1, or equivalently x³+2x²+7x+3. a. what...
- E English The section of a report that includes the body of the report. 2 points theme record source Detail Field List pane...
- M Mathematics Abakery offers a sales 2.85 for 4 muffins what is the price per dozen...
- M Mathematics You want to test the claim that the average age of students at Gorka College is greater than the average age of students at Yapoah College. You take a simple random sample of...
- M Mathematics What is the greatest whole-number remainder if you divide any number by 41? explain....

Ответ:
During the seventeenth and especially eighteenth centuries, driven both by a desire to understand nature and a quest to make balloons in which they could fly (Figure 1), a number of scientists established the relationships between the macroscopic physical properties of gases, that is, pressure, volume, temperature, and amount of gas. Although their measurements were not precise by today’s standards, they were able to determine the mathematical relationships between pairs of these variables (e.g., pressure and temperature, pressure and volume) that hold for an ideal gas—a hypothetical construct that real gases approximate under certain conditions. Eventually, these individual laws were combined into a single equation—the ideal gas law—that relates gas quantities for gases and is quite accurate for low pressures and moderate temperatures. We will consider the key developments in individual relationships (for pedagogical reasons not quite in historical order), then put them together in the ideal gas law.
Hope this helps!
(☞゚ヮ゚)☞ ÙwÚ