limelight11
17.12.2019 •
Chemistry
Commercial cold packs consist of solid nh4no3 and water. in a coffee-cup calorimeter, 5.60g nh4no3 is dissolved in 100g of water at 22.0c; the temperature falls to 17.9c. assuming that the specific heat capacity of the solution is 4.18 j/(g*k), calculate the enthalpy of dissolution of nh4no3, in kj/mol.
Solved
Show answers
More tips
- F Food and Cooking Discover the Benefits and Properties of Dates...
- C Computers and Internet Dynamically Assigned IP Address: What Is It and How Does It Work?...
- H Health and Medicine Angina: Causes, Symptoms, and Treatment...
- C Computers and Internet How to Learn to Type Fast?...
- F Food and Cooking Delight for Gourmets: How to Prepare Liver Pate...
- S Style and Beauty How to braid friendship bracelets?...
- H Health and Medicine Mercury Thermometer Danger: What to do when a thermometer breaks?...
- F Food and Cooking Which Calamari Salad is the Most Delicious?...
- S Society and Politics 10 Tips for Boosting Your Self-Esteem...
- F Food and Cooking The Most Delicious and Simple Fish in Batter Recipe...
Answers on questions: Chemistry
- C Chemistry If the ph of a 1.00-in. rainfall over 1800 miles2 is 3.70, how many kilograms of sulfuric acid, h2so4, are present, assuming that it is the only acid contributing...
- C Chemistry How many chloride ions are in a 220 grams of calcium chloride?...
- C Chemistry Why do you think reducing the amount of acid in the stomach would to heal stomach ulcers...
- C Chemistry Δs is negative for the reaction a.2so2 (g) + o2 (g) → 2so3 (g) b.nh4cl (s) → nh3 (g) + hcl (g) c.pbcl2 (s) → pb2+ (aq) + 2cl- (aq) d.2c (s) + o2 (g) → 2co2 (g)...
- C Chemistry What is responsible for the unusual chemical properties of water?...
- C Chemistry draw the structure of the major organic product of the following reaction. predict whether the product will be an aldol or an enone....
- C Chemistry a 2-dimensional 3x3 array of ints, has been created and assigned to tictactoe. write an expression whose value is that of the first element in the first row....
- C Chemistry Suggest one reason why you would not look at your own blood cells blood cells in the classroom...
- C Chemistry How does using more water in a beaker affect the solubility in an experiment?...
- C Chemistry How are Distilled water and tap water alike?...

Ответ:
-1.37 kJ/mol
Explanation:
The expression for the calculation of the enthalpy of dissolution of [tex[NH_4NO_3[/tex] is shown below as:-
Where,
m is the mass
C is the specific heat capacity
Thus, given that:-
Mass of ammonium nitrate = 5.60 g
Specific heat = 4.18 J/g°C
So,
Negative sign signifies loss of heat.
Also, 1 J = 0.001 kJ
So,
Also,
Molar mass of [tex[NH_4NO_3[/tex] = 80.043 g/mol
The formula for the calculation of moles is shown below:
Thus,
Thus,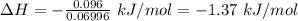
Ответ:
jjj and I will also need the olympics is there a way for