Consider the first-order reaction described by the equation Cyclopropane gas isomerizes to propene gas. At a certain temperature, the rate constant for this reaction is 5.05 × 10 − 4 s − 1 . Calculate the half-life of cyclopropane at this temperature. t 1 / 2 = s Given an initial cyclopropane concentration of 0.00670 M , calculate the concentration of cyclopropane that remains after 2.00 hours. concentration.
Solved
Show answers
More tips
- F Family and Home Why Having Pets at Home is Good for Your Health...
- H Health and Medicine How to perform artificial respiration?...
- H Health and Medicine 10 Tips for Avoiding Vitamin Deficiency...
- F Food and Cooking How to Properly Cook Buckwheat?...
- F Food and Cooking How Many Grams Are In a Tablespoon?...
- L Leisure and Entertainment Carving: History and Techniques for Creating Vegetable and Fruit Decorations...
- P Photography and Videography How to Choose the Perfect Photo Paper for Your Images?...
- H Health and Medicine What vaccines do children need?...
- H Health and Medicine Reasons for the Appearance of Warts: Everything You Need to Know...
- A Art and Culture How to Learn Screaming: Step-by-Step Guide for Beginners...
Answers on questions: Chemistry
- C Chemistry What is the scientifik method...
- E English Any help would be greatly appreciated. 3...
- A Arts Why would creativity be needed in designing products like this car?...
- L Law Lester is stopped on a public highway and he has a shell in the guns magazine but not in the chamber has he violated the fish and game code true or false...

Ответ:
The half-life would be 1372 seconds.
The concentration remains after 2 hours is 0.000177 M.
Explanation:
Given the initial concentration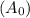 of was
of was  .
.
And the rate constant is
is 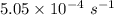
We need to calculate the half-life and the concentration remains after 2 hours.
after 2 hours.
First, we will use the formula of half-life of the first order reaction.
So, half-life would be 1372 seconds.
Now, we will find its concentration after 2 hours. That means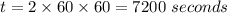
Use the equation
So, the concentration remains after 2 hours is 0.000177 M.
Ответ:
allegro,od em
Step-by-step explanation:
anyways historia much 2 plus to lets do it 4 lol