Consider the reaction directly below and answer parts a and b. c6h4(oh)2 (l) + h2o2 (l) à c6h4o2 (l) + 2 h2o (l)
(a) use the following information to calculate dh° for the above reaction. show all work. dh° c6h4(oh)2 (l) à c6h4o2 (l) + h2 (g) +177.4 kj h2 (g) + o2 (g) à h2o2 (l) -187.8 kj h2 (g) + o2 (g) à h2o (l) -285.8 kj
(b) based on your answer to part a, above, would heat be gained or lost by the surroundings as the reaction at the very top of the page occurred at standard conditions?
(c) how many kilojoules of heat are given off when 20.0 g of h2o (l) are produced at standard conditions according to the reaction at the very top of the page? show all work.
Solved
Show answers
More tips
- O Other Everything You Need to Know About Kudyabliks...
- C Computers and Internet The Twitter Phenomenon: What it is and How to Use it...
- C Computers and Internet How to Choose a Laptop: Expert Guide and Tips...
- C Computers and Internet How to Choose a Monitor?...
- H Horoscopes, Magic, Divination Where Did Tarot Cards Come From?...
- S Style and Beauty How to Make Your Lips Fuller? Ideas and Tips for Beautiful Lips...
- C Computers and Internet How to Learn to Type Fast?...
- A Art and Culture Who Said The Less We Love a Woman, the More She Likes Us ?...
- F Family and Home How to Get Rid of Your Neighbors?...
Answers on questions: Chemistry
- C Chemistry Which scenario would produce a quicker rate of reaction? Reaction occurring at 0 C Reaction occuring at 20 There is no way to know without doing carrying out the reaction...
- C Chemistry Which of the following substances can be dissolved in pure water to give a basic solution? Hydrogen chloride Sodium bromide Sodium hydroxide Sodium chloride...
- C Chemistry A flower with orange-spotted petals was grown from a seed. The flower was allowed to self-pollinate; self-pollination occurs when pollen is transferred from the anther to...
- C Chemistry Properties of substances can be classified as physical or chemical properties. which statements describe chemical properties? (choose all that apply) a. a magnet is attracted...
- C Chemistry Which characteristic of life is demonstrated when eagles use sharp talons to grab fish from the water? a. acquiring energy b. reproducing c. maintaining structure d. instinct...
- C Chemistry Give the major product formed when phenol is heated with concentrated H2SO4 and SO3. (There is more than one correct answer, but draw just one.)...
- M Mathematics VERTICIES AND COORDINATES A(2,2), B(2, 6), C(5, 2) I VE BEEN WORKING IT FOR 6 HOURS STRAIGHT HELP ME....
- M Mathematics Wo similar triangles are shown on the coordinate grid: A coordinate plane is shown. Triangle ABC has vertices A at negative 3 comma 2, B at negative 1 comma 0, and C at...
- M Mathematics At the beginning of the day, there are 64 ounces of water in Todd s water bottle. At the end of the day, there are 0 ounces of water in his bottle. What integer represents...
- E English Part 2: Explain the theme to Scrooge , and how did the character change throughout the story?...

Ответ:
a. -206,4kJ
b. Surroundings will gain heat.
c. -115kJ are given off.
Explanation:
It is possible to obtain ΔH of a reaction using Hess's law that consist in sum the different ΔH's of other reactions until obtain the reaction you need.
Using:
(1) C₆H₄(OH)₂(l) → C₆H₄O₂(l) + H₂(g) ΔH: +177.4 kJ
(2) H₂(g) + O₂(g) → H₂O₂ (l) ΔH: -187.8 kJ
(3) H₂(g) + 1/2O₂(g) → H₂O(l) ΔH: -285.8 kJ
It is possible to obtain:
C₆H₄(OH)₂(l) + H₂O₂(l) → C₆H₄O₂(l) + 2H₂O(l)
From (1)-(2)+2×(3). That is:
(1) C₆H₄(OH)₂(l) → C₆H₄O₂(l) + H₂(g) ΔH: +177.4 kJ
-(2) H₂O₂(l) → H₂(g) + O₂(g) ΔH: +187.8 kJ
2x(3) 2H₂(g) + O₂(g) → 2H₂O(l) ΔH: 2×-285.8 kJ
The ΔH you obtain is:
+177,4kJ + 187,8kJ - 2×285.8 kJ = -206,4kJ
b. When ΔH of a reaction is <0, the reaction is exothermic, that means that the reaction produce heat and the surroundings will gain this heat.
c. 20,0g of H₂O are:
20,0g×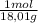 = 1,11 mol H₂O
= 1,11 mol H₂O
As 2 moles of H₂O are produced when -206,4kJ are given off, when 1,11mol of H₂O are produced, there are given off:
1,11mol H₂O×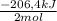 = -115kJ
= -115kJ
I hope it helps!
Ответ:
G–T–A–G–C–T
Explanation: