magaaalimartinez
02.07.2019 •
Chemistry
Consider the two reactions. 2nh3(g)+3n2o(g)4nh3(g)+3o2(g)⟶4n2(g)+3h2o(l)⟶2n2(g)+6h2o(l) δ∘=−1010 kjδ∘=1531 kj using these two reactions, calculate and enter the enthalpy change for the reaction below. n2(g)+12o2(g)⟶n2o(g)
Solved
Show answers
More tips
- A Auto and Moto How to choose the right drive for your BMW...
- L Leisure and Entertainment How to Choose the Perfect Gift for Men on February 23rd?...
- H Health and Medicine How to Treat Whooping Cough in Children?...
- H Health and Medicine Simple Ways to Lower Cholesterol in the Blood: Tips and Tricks...
- O Other How to Choose the Best Answer to Your Question on The Grand Question ?...
- L Leisure and Entertainment History of International Women s Day: When Did the Celebration of March 8th Begin?...
- S Style and Beauty Intimate Haircut: The Reasons, Popularity, and Risks...
- A Art and Culture When Will Eurovision 2011 Take Place?...
- S Style and Beauty How to Choose the Perfect Hair Straightener?...
- F Family and Home Why Having Pets at Home is Good for Your Health...
Answers on questions: Chemistry
- C Chemistry While heating two different samples of water at sea level, one boils at 102°C and one boils at 99.2°C. Calculate the percent error for each sample from the theoretical 100.0°C....
- C Chemistry Gravity is responsible for the Orbiting path of the panets around the sun. Rotation of a planet on its rotation axis. Tilt of Earth s axis. Pheses of the moon....
- C Chemistry 30.) What charge would you expect from a Group 16 atom when it becomes an ion? a.) 1- b.) 2+ c.) 1+ d.) 2-...
- C Chemistry ASAP: The gases in a can of compressed air are at a temperature of 274.9 K and a pressure of 227.2 kPa. If the gases in the can reach a pressure of 845.3 kPa, the can will explode....
- C Chemistry Calculate the molar enthalpy of formation of 1 mole of PbSO4(s) from its elements, given the following equations: Pb(s) + S(s) → PbS(s) + 94 kJ/mol PbS(s) + 2O2(g) → PbSO4(s) +...
- C Chemistry Protons are blank charged particles...
- C Chemistry How does insect damage to crops in kenya compare to insect damage to crops in north america...
- M Mathematics Please help me with #24...
- S Social Studies How was Prithvi Narayan Shah in terms of democracy? Explain your answer based on his statement, “Ministers should be appointed according to the will of the people” and his decision...
- C Computers and Technology Reciprocal of 2/3+4/5 please send me answer...

Ответ:
The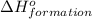 for the reaction is 591.9 kJ.
for the reaction is 591.9 kJ.
Explanation:
Hess’s law of constant heat summation states that the amount of heat absorbed or evolved in a given chemical equation remains the same whether the process occurs in one step or several steps.
According to this law, the chemical equation is treated as ordinary algebraic expressions and can be added or subtracted to yield the required equation. This means that the enthalpy change of the overall reaction is equal to the sum of the enthalpy changes of the intermediate reactions.
The chemical reaction for the formation reaction of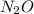 is:
is:
The intermediate balanced chemical reaction are:
(1)
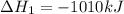 ( ÷ 3)
( ÷ 3)
(2)![4NH_3(g)+3O_2(g)\rightarrow 2N_2(g)+6H_2O(l) [tex]\Delta H_2=1531kJ](/tpl/images/0041/0429/f3925.png) ( ÷ 6)
( ÷ 6)
Reversing Equation 1 and then adding both the equations, we get the enthalpy change for the chemical reaction.
Putting values in above equation, we get:
Hence, the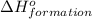 for the reaction is 591.9 kJ.
for the reaction is 591.9 kJ.
Ответ:
A) -5
Answer to the 2nd question: the linear factors of the cubic are: A) (x-2)(x-3)(x-5)
Answer to the 3rd question: to solve for the leading coefficient, use: D) -5=a(0-2)(0-3)(0-5)
Answer to the 4th question: A=1/6
Answer to the 5th question: write the function: f(x)=1/6 x^3-=5/3 x^2+=31/6 x-=5