camrynhelm7193
10.03.2020 •
Chemistry
Consider these reactions, where M represents a generic metal. 2 M ( s ) + 6 HCl ( aq ) ⟶ 2 MCl 3 ( aq ) + 3 H 2 ( g ) Δ H 1 = − 760.0 k J HCl ( g ) ⟶ HCl ( aq ) Δ H 2 = − 74.8 k J H 2 ( g ) + Cl 2 ( g ) ⟶ 2 HCl ( g ) Δ H 3 = − 1845.0 k J MCl 3 ( s ) ⟶ MCl 3 ( aq ) Δ H 4 = − 267.0 k J Use the given information to determine the enthalpy of the reaction 2 M ( s ) + 3 Cl 2 ( g ) ⟶ 2 MCl 3 ( s )
Solved
Show answers
More tips
- H Health and Medicine How to Calculate Your Ideal Weight?...
- S Style and Beauty Discover the Art of Nail Design: How Do You Paint Your Nails?...
- P Philosophy How to Develop Extrasensory Abilities?...
- O Other Everything You Need to Know About Kudyabliks...
- C Computers and Internet The Twitter Phenomenon: What it is and How to Use it...
- C Computers and Internet How to Choose a Laptop: Expert Guide and Tips...
- C Computers and Internet How to Choose a Monitor?...
- H Horoscopes, Magic, Divination Where Did Tarot Cards Come From?...
- S Style and Beauty How to Make Your Lips Fuller? Ideas and Tips for Beautiful Lips...
- C Computers and Internet How to Learn to Type Fast?...
Answers on questions: Chemistry
- C Chemistry 4) comparing algae and plants single celled algae multicelullar algae multicellular green plant all living things are made of one or more cells. how does the green plant in slide...
- C Chemistry Whice type of protein stays only on the surface of the plasma membrane...
- C Chemistry What is the number of each type of atom on the right side of the equation 2na3po4(aq)+2cocl2(aq)→2co3(po4)2(s)+6nacl(aq)...
- C Chemistry Why does topsoil have a dark color)...
- C Chemistry Asolution is prepared by dissolving 106.3 g hcl(g) in enough water to make 175.0 l of solution. the ph of this solution is...
- C Chemistry Pls will give crown to best answer! 20 points! for metals, reactivity as you go from in a period. a. increases; left to right b. decreases; left to right c. increases; top to...
- C Chemistry Does the mass of the sealed jar and its contents change upon the vaporization of the liquid?...
- C Chemistry Layers of rock that bend can produce a downward fold known as a(n)...
- C Chemistry Electrically charged atoms or combinations of atoms are called...
- C Chemistry Why is it important to use low flame when evaporating water from a recovered filtrate?...

Ответ:
Answer : The enthalpy of the reaction is, -6209.8 kJ
Explanation :
According to Hess’s law of constant heat summation, the heat absorbed or evolved in a given chemical equation is the same whether the process occurs in one step or several steps.
According to this law, the chemical equation can be treated as ordinary algebraic expression and can be added or subtracted to yield the required equation. That means the enthalpy change of the overall reaction is the sum of the enthalpy changes of the intermediate reactions.
The Main reaction is:
The intermediate balanced chemical reaction will be,
(1)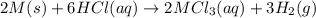
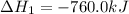
(2)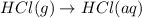

(3)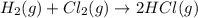
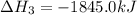
(4)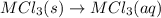
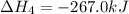
We are multiplying equation 3 by 3, reverse reaction of 4 by 2 and reaction 2 by 6 and then adding all the equations, we get :
(1)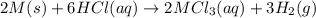
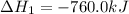
(2)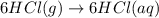
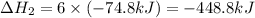
(3)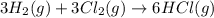
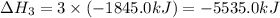
(4)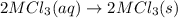
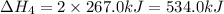
The expression for enthalpy of the reaction is,
Therefore, the enthalpy of the reaction is, -6209.8 kJ
Ответ:
calcite and fluorite
Explanation:
I hope it will help u dear