Copper(ii) sulfate forms a bright blue hydrate with the formula cuso 4 ⋅ n h 2 o ( s ) . if this hydrate is heated to a high enough temperature, h 2 o ( g ) can be driven off, leaving the grey‑white anhydrous salt cuso 4 ( s ) . a 14.220 g sample of the hydrate was heated to 300 ∘ c . the resulting cuso 4 ( s ) had a mass of 8.9935 g . calculate the val
Solved
Show answers
More tips
- S Style and Beauty Tricks and Tips: How to Get Rid of Freckles...
- H Health and Medicine What is Autism? Understanding the Basics of This Neurodevelopmental Disorder...
- F Food and Cooking The Most Delicious and Simple Fish in Batter Recipe...
- S Society and Politics 10 Tips for Boosting Your Self-Esteem...
- F Food and Cooking Which Calamari Salad is the Most Delicious?...
- H Health and Medicine Mercury Thermometer Danger: What to do when a thermometer breaks?...
- S Style and Beauty How to braid friendship bracelets?...
- F Food and Cooking Delight for Gourmets: How to Prepare Liver Pate...
- C Computers and Internet How to Learn to Type Fast?...
- H Health and Medicine Angina: Causes, Symptoms, and Treatment...
Answers on questions: Chemistry
- C Chemistry 16) 5AI(C2H2O2)2 # of molecules: # of elements: Name of element: # of atoms: Total # of atoms: The #2 is a (coefficient or subscript)...
- C Chemistry Explain why a reaction that is spontaneous in one direction is non-spontaneous in the reverse direction...
- C Chemistry Choose the combination of factors that creates fog. Record the description of the factors in the Student Guide, Relative Humidity Air Temperature Air Pressure Check Pause...
- C Chemistry What areas of the world would a tidal-power-generating facility be viable? I couldn’t find the answer at all, can someone please help me?...
- C Chemistry Assume the reaction coordinate diagram above is for the reaction A+B = C+D. Also let’s assume the following: 50 particles of A collide with 50 particles of B with 100 kl of energy....
- C Chemistry Two students prepare two cyclohexane solutions having the same freezing point. Student 1 uses 26.6 g of cyclohexane solvent, and student 2 uses 24.1 g of cyclohexane solvent....
- C Chemistry A 0.194-g sample of a nonvolatile solid solute dissolves in 9.82 g of cyclohexane. The change in the freezing point of the solution is 2.94°C. (Kf of Cyclohexane is 20.0°C/m)...
- C Chemistry What volume of 18 m sulfuric acid must be used to prepare 1.80 l of 0.215 m h2so4?...
- C Chemistry Calculate the mass of naoh needed to prepare 100. ml of 0.15m solution....
- C Chemistry How do isotopes affect an element s average atomic mass?...

Ответ:
The value of n in the hydrate formula is 5 ,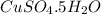 .
.
Explanation:
Mass of hydrate of copper sulfate = 14.220 g
Moles of hydrate of copper sulfate =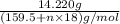
Mass of copper sulfate after heating = 8.9935 g
Moles of copper sulfate =
Solving for n, we get:
n = 5
The value of n in the hydrate formula is 5 ,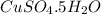 .
.
Ответ:
NameFormula and Charge NameFormula and Charge
ammoniumNH4+ hydroxideOH−
acetateC2H3O2−, or CH3COO−nitrateNO3−
bicarbonate (hydrogen carbonate)HCO3−nitriteNO2−
bisulfate (hydrogen sulfate)HSO4−peroxideO22−
carbonateCO32−perchlorateClO4−
chlorateClO3−phosphatePO43−
chromateCrO42−sulfateSO42−
cyanideCN−sulfiteSO32−
dichromateCr2O72−triiodideI3−
Explanation: