mallorytaylor279
29.07.2019 •
Chemistry
Energy of sublimation of na(s) = 97 kj/mol bond energy of hbr = 363 kj/mol ionization energy of na(g) = 496 kj/mol electron affinity of br(g) = –324 kj/mol lattice energy of nabr(s) = –781 kj/mol bond energy of h2 = 432 kj/mol calculate the net change in energy for the following reaction: 2na(s) + 2hbr(g) → 2nabr(s) + h2(g)
Solved
Show answers
More tips
- L Leisure and Entertainment The Best Film of 2010: A Look Back at the Academy Awards...
- H Health and Medicine Simple and Effective: How to Get Rid of Cracked Heels...
- L Leisure and Entertainment What to Bring on a Hike? Essential Items to Pack for a Safe and Enjoyable Adventure...
- L Leisure and Entertainment Couchsurfing: A New Way to Travel...
- S Style and Beauty Autotanning: Harmful or Safe?...
- F Food and Cooking 10 Ideas for a Wedding Anniversary Gift...
- H Health and Medicine How to Reduce Sweating in the Heat and Beyond: Say Goodbye to Excessive Sweat...
- F Food and Cooking Do Aphrodisiacs Really Work? Separating Fact from Fiction...
- H Health and Medicine What to Eat to Lose Weight?...
- A Animals and plants How to Teach Your Parrot to Talk?...
Answers on questions: Chemistry
- E English The words jump and hop would be considered . The words jump and hop would be considered . 5 points denotations synonyms connotations antonyms...
- B Business Why do lags make if difficult to fine tune the economy?...
- S Social Studies Why would many reformers have been unsatisfied with the Fifteenth Amendment?...
- E English A toddler s ability to hold a spoon is categorized as which of the following...
- B Biology If negative consequences still persist what possible solutions might there be fo fix the problems of the printing oress...

Ответ:
Answer : The concentration can be expressed as, 1.99 M, or 15.1(m/m)%, or 18(m/v)%
Solution : Given,
Volume of solution = 0.5 L = 500 ml (1 L = 1000 ml)
Mass of solution = 596 g
Mass of oxalic acid, (solute) = 90 g
Molar mass of oxalic acid = 90.03 g/mole
First we have to calculate the concentration in terms of M(molarity).
Molarity : Molarity is defined as the number of moles of solute present in one liter of solution.
Formula used :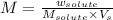
where,
M = molarity of solution
Now put all the given values in this formula, we get
Now we have to calculate the concentration in terms of (m/m)%
(m/m)% : It is defined as the mass of solute present in mass of solution in grams.
Formula used :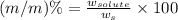
where,
Now we have to calculate the concentration in terms of (m/v)%
(m/v)% : It is defined as the mass of solute present in one milliliter of solution.
Formula used :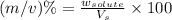
where,