love123jones
23.10.2019 •
Chemistry
Enter your answer in the provided box. roasting galena [lead(ii) sulfide] is an early step in the industrial isolation of lead. how many liters of sulfur dioxide, measured at stp, are produced by the reaction of 5.05 kg of galena with 123 l of oxygen gas at 220°c and 2.00 atm
Solved
Show answers
More tips
- F Food and Cooking Discover the most delicious spaghetti with these tips...
- P Philosophy How did the concept of module arise in computer science?...
- F Food and Cooking How to Cook Julienne? Recipes and Tips...
- H Health and Medicine At What Age Does a Person Stop Growing?...
- F Family and Home How to Choose a Name for Your Baby?...
- F Food and Cooking Discover the Health Benefits of Cedar Nuts...
- L Leisure and Entertainment Scrapbooking: What is it and Why is it Becoming More Popular?...
- L Leisure and Entertainment Carving: History and Techniques for Creating Vegetable and Fruit Decorations...
- F Food and Cooking How to Make Sushi: A Step-by-Step Guide to Perfectly Rolled Delights...
- T Travel and tourism Maldives Adventures: What is the Best Season to Visit the Luxurious Beaches?...
Answers on questions: Chemistry
- C Chemistry LICIUL Consider this reaction mechanism: Step 1: Mo(CO)6 » Mo(CO)3 + CO Step 2: Mo(CO)3 + P(CH3)3 - Mo(CO)P(CH3)3 In terms of molecularity, Step 1 is and Step 2...
- C Chemistry Thermodynamics is the study of the movement of thermal energy (heat). Why do we study heat in a chemistry course?...
- C Chemistry What is listed on the right side of a chemical equation...
- C Chemistry A, 3B, and 7A are examples of on the periodic table....
- C Chemistry 4Fe + 3O2 + xH2O → 2Fe2O3 • xH2O The formation of rust (equation above) is an example of both combustion and...
- C Chemistry Gobble me, swallow me, drip down the side of me (yeah) Quick, jump out fore you let it get inside of me (yeah) I tell him where to put it, never tell him where...
- H History What types of trials did freedmen face after emancipation?...
- S Social Studies Which of the following buffalo parts was stretched out to create bullboats? a. hooves b. hide c. gristle d. horns...
- P Physics At which position does the diver have the most kinetic energy...
- M Mathematics What is the selling price if the cost is $22.00 and the markup is $6.80...

Ответ:
Answer : The volume of produced are 90.7 liters.
produced are 90.7 liters.
Explanation :
The balanced chemical reaction will be:
First we have to calculate the moles of PbS.
Molar mass of PbS = 239.26 g/mole
Now we have to calculate the moles of by using ideal gas equation.
by using ideal gas equation.
Using ideal gas equation :
where,
P = Pressure of gas = 2.00 atm
gas = 2.00 atm
V = Volume of gas = 123 L
gas = 123 L
n = number of moles = ?
= ?
R = Gas constant =
T = Temperature of gas =
gas = 
Putting values in above equation, we get:
The number of moles of is, 6.07 mole
is, 6.07 mole
Now we have to calculate the limiting and excess reagent.
From the balanced reaction we conclude that
As, 3 mole of react with 2 mole of
react with 2 mole of 
So, 6.07 moles of react with
react with 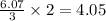 moles of
moles of 
From this we conclude that, is an excess reagent because the given moles are greater than the required moles and
is an excess reagent because the given moles are greater than the required moles and  is a limiting reagent and it limits the formation of product.
is a limiting reagent and it limits the formation of product.
Now we have to calculate the moles of
From the reaction, we conclude that
As, 3 mole of react to give 2 mole of
react to give 2 mole of 
So, 6.07 moles of react to give
react to give 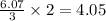 moles of
moles of 
Now we have to calculate the volume of produced at STP.
produced at STP.
As, 1 mole of contains 22.4 L volume of
contains 22.4 L volume of 
So, 4.05 mole of contains
contains  volume of
volume of 
Therefore, the volume of produced are 90.7 liters.
produced are 90.7 liters.
Ответ:
the must be able to see what happened and give all the details
Explanation: