mairadua14
19.02.2020 •
Chemistry
Fermentation of 826 mL grape juice (density is 1.0 ) is allowed to take place in a bottle with a total volume of 885 mL until 19% by volume is ethanol (). Assuming that obeys Henry’s law, calculate the partial pressure of in the gas phase and the solubility of in the wine at 25°C. The Henry’s law constant for is mol/L ⋅ atm at 25°C with Henry’s law in the form , where is the concentration of the gas in mol/L. (The density of ethanol is 0.79 .)
Solved
Show answers
More tips
- W Work and Career Everything You Need to Know About MBA Programs...
- S Sport How to Do Push-ups Correctly?...
- S Style and Beauty How to Grow Hair Faster: Real Methods and Advice...
- F Family and Home How to Remove Fading from Clothes: Tips and Tricks...
- F Food and Cooking How to Make Polendwitsa at Home?...
- F Family and Home Parents or Environment: Who Has the Most Influence on a Child s Upbringing?...
- P Philosophy Unbelievable stories of encounters with otherworldly forces...
- L Leisure and Entertainment How to Choose the Perfect Gift for Men on February 23rd?...
- H Health and Medicine How to Treat Whooping Cough in Children?...
- H Health and Medicine Simple Ways to Lower Cholesterol in the Blood: Tips and Tricks...
Answers on questions: Chemistry
- C Chemistry Which group on the Periodic Table most likely contains an element that is highly reactive, a good conductor of electricity, and malleable?...
- C Chemistry Why Ammonia shows basic nature?...
- C Chemistry HURRY TAKING TEST Which situation is the best example of translational motion? an ice skater spinning in place a soccer ball passed between two players water molecules...
- C Chemistry Can y’all help me? btw Merry Christmas!...
- C Chemistry hi :) I need help with how to write the Full electron configuration, the noble gas configuration , valence electron and lewis dot notation. I’m having trouble understanding...
- C Chemistry How many atoms are in 5.0 moles of lithium?...
- C Chemistry Calcium Chloride is sometimes used as a de-icer. Calculate the percent by mass of each element in CaCl2...
- C Chemistry A. Which of the following statements about empirical formulas is incorrect? An empirical formula represents a molecule. b. An empirical formula gives the smallest...
- C Chemistry 9. Zinc and phosphorus react to give zinc phosphide. 9.75 g of zinc combines with 3.1 g of phosphorus a. Find the empirical formula for the compound. (Ar.: Zn =...
- C Chemistry How do you think dry cleaning works?...

Ответ:
Here is the full and correct question
In the "Methode Champenoise", grape juice is fermented in a wine bottle to produce sparkling wine. The reaction is:
C₆H₁₂O₆(aq) -----------> 2C₂H₅OH(aq) + 2CO₂(g)
Fermentation of 826 mL grape juice (density is 1.0 g/cm³) is allowed to take place in a bottle with a total volume of 885 mL until 19% by volume is ethanol(C₂H₅OH).
Assuming that:
CO₂ obeys Henry's Law, calculate the partial pressure of CO₂ in the gas phase and the Solubility of CO₂ in the wine at 25°C
The Henry Law Constant for CO₂ is 3.1 × 10⁻² mol/L. atm at 25°C with Henry's Law in the form C = KP;
where:
C = concentration of the gas in mol/L.
(The solubility of ethanol is 0.79 g/cm³)
Partial Pressure of CO₂ in the gas phase is 104.84 atm
Solubility of CO₂ in the wine at 25°C is 3.25004 M
Explanation:
The equation for the reaction is given as:
C₆H₁₂O₆(aq) -----------> 2C₂H₅OH(aq) + 2CO₂(g)
Given that:
The total volume = 885 mL
Volume of ethanol is by 19% of the total volume = 0.19 × 885
Density = 0.79
density =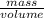
the mass of ethanol can be calculated as = 0.79 × 0.19 × 885 = 132.84 g
number of moles of ethanol =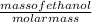
molar mass of ethanol = 46.07
∴
number of moles of ethanol =
= 2.8834 mole
Henry's Law posits that the solubility of a gas is directly proportional to the partial pressure of the gas above the solution.
NOW, Assuming that CO₂ obeys Henry's Law;
then numbers of moles of ethanol = numbers of mole of CO₂
So, molar mass of CO₂ = 44.01
then mass of CO₂ = number of moles of CO₂ × molar mass
mass of CO₂ = 2.8834 mole × 44.01
mass of CO₂ = 126.90 g
Since total volume = 885 mL = 0.885 m
Concentration of CO₂ =
Concentration of CO₂ =
Concentration of CO₂ = 3.258 M
C =
P =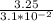
P = 104.84 atm
∴ the partial pressure of in the gas phase = 104.84 atm
b)
Solubility of ethanol in the wine =
Solubility of ethanol in the wine =
Solubility of ethanol in the wine = 3.25004 M
Ответ: