PlsHelpMeh3401
21.01.2020 •
Chemistry
Ferrophosphorus (fe2p) reacts with pyrite (fes2) producing iron(ii) sulfide and a compound that is 27.87% p and 72.13% s by mass and has a molar mass of 444.56 g/mol.
a. determine the empirical and molecular formulas of this compound.
b. empirical formula: molecular formula:
c. write a balanced chemical equation for this reaction. do not include phases.
Solved
Show answers
More tips
- C Computers and Internet How to Choose an Uninterruptible Power Supply (UPS) for Your Computer: Expert Tips...
- F Food and Cooking The Health Benefits of Flaxseed oil...
- S Style and Beauty Why is Sugaring Better than Waxing for Hair Removal?...
- W Work and Career Where can you learn to be a flight attendant?...
- G Goods and services How to Properly Calculate the Power of Your Air Conditioner?...
- F Food and Cooking Effective Methods to Organize Videos in your iPad According to Content...
- F Family and Home Parquet or laminate, which is better?...
- L Leisure and Entertainment How to Properly Wind Fishing Line onto a Reel?...
- L Leisure and Entertainment How to Make a Paper Boat in Simple Steps...
- T Travel and tourism Maldives Adventures: What is the Best Season to Visit the Luxurious Beaches?...
Answers on questions: Chemistry
- C Chemistry How many grams of HNO, are in 1.655 x 1022 molecules of HNO,? Show the conversions required to solve this problem and calculate the grams of HNO3. 63.018 g HNO,...
- C Chemistry Which of these is a chemical property?...
- H History Which geographic features impacted the development of Greece? A) Rivers flooded routinely but valleys gave fertile land for farming B) The cold climate made farming...
- M Mathematics (0,-9);slope=4 (3,-8);slope=-3 (-4,-7);slope=1 (4,9);slope=5 (-6,-8);slope=5/6 (7,-8);slope= -2 I need each problem down with step by step....
- M Mathematics I HOPE YOU CAN HELP ME ON MATH TEST...
- M Mathematics PLEASE ANSWER ASAP! THANK YOUU SOO MUCH! What is the equation of a line, in point- slope form, that passes through (5, –3)and has a slope of 2/3? A. y+3= 2/3 (x-5)...
- M Mathematics What’s the domain and range? Tickets to a sporting event cost $125 each....
- M Mathematics Please help I don’t understand...
- E English Plz help with the 2 questions. I ll mark you brainliest...
- H History At the beginning of the Depression, African Americans who managed to obtain aid usually got white people. Select the best answer from the choices provided. A. the...

Ответ:
The molecular formula of the compound =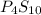
The empirical formula of the compound =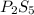
The balanced chemical equation for this reaction:
Explanation:
Compound that is 27.87% P and 72.13% S by mass and has a molar mass of 444.56 g/mol.
Molar mass of compound = 444.56 g/mol
Number of phosphorus atom = x
Number of sulfur atom = y
Atomic mass of phosphorus 31 g/mol
Atomic mass of sulfur = 32 g/mol
Percentage of element in compound :
Phosphorus :
x = 4
Sulfur :
y = 10
The molecular formula of the compound =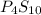
Empirical formula is the simplest chemical formula which depicts the whole number of atoms of each element present in the compound.
The empirical formula of the compound =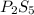
The balanced chemical equation for this reaction:
Ответ:
what kinda school u go to lolll