elyssa34972
24.09.2019 •
Chemistry
For a gas to be soluble in water, the gas should contain (nonpolar, polar ) molecules. the water should be at a (high, low) temperature, and the gas should be at (high, low ) pressure above the surface of the water.
Solved
Show answers
More tips
- A Auto and Moto How to choose the right drive for your BMW...
- L Leisure and Entertainment How to Choose the Perfect Gift for Men on February 23rd?...
- H Health and Medicine How to Treat Whooping Cough in Children?...
- H Health and Medicine Simple Ways to Lower Cholesterol in the Blood: Tips and Tricks...
- O Other How to Choose the Best Answer to Your Question on The Grand Question ?...
- L Leisure and Entertainment History of International Women s Day: When Did the Celebration of March 8th Begin?...
- S Style and Beauty Intimate Haircut: The Reasons, Popularity, and Risks...
- A Art and Culture When Will Eurovision 2011 Take Place?...
- S Style and Beauty How to Choose the Perfect Hair Straightener?...
- F Family and Home Why Having Pets at Home is Good for Your Health...
Answers on questions: Chemistry
- C Chemistry Im tired of waking up every morning i want to sleep foreverrr...
- C Chemistry A student placed a disk magnet on top of the battery to make a simple motor. When the coil was given a gentle spin it kept turning on its own. Which stamens best explains...
- C Chemistry The specific heat of gold is 0.031 calories/gram°c and the specific heat of silver is 0.057 calories/gram°c. if equal amounts of each metal are exposed to equal heating,...
- C Chemistry Which statements explain the shape of the titration curve observed in lab? i. initially, the conductivity is zero since the reaction has not begun. ii. during the reaction,...
- C Chemistry An individual picks up a cup and drinks from it. this is an example of a. a concentric contraction because muscles cause the elbow joint and arm to move b. an eccentric contraction...
- C Chemistry Consider the reaction 2 x2y + z2 ⇌ 2 x2yz which has a rate law of rate= k[x2y][z2] select a possible mechanism for the reaction. group of answer choices step 1: z2 -- z +...
- C Chemistry Vitamin , cyanocobalamin, is essential for human nutrition. it is concentrated in animal tissue but not in higher plants. although nutritional requirements for the vitamin...
- C Chemistry Draw schematically the diffusion coefficient d in a log scale versus 1/t (t is temperature in kelvin) for diffusion (i) within crystal bulk, (ii) along grain boundaries,...
- C Chemistry Space shuttle tiles, sandstone, and the glass in quartz halogen lamps are all made out of the same stuff: silicon dioxide. why do the three materials have such different...
- C Chemistry Sketch the key components of a ph combination electrode and very briefly describe in your own words how it works and how does one use it to determine the ph of a solution....

Ответ:
The gas should contain polar molecules. The water should be at a low temperature, and the gas should be at high pressure above the surface of the water.
Explanation:
Water is a dipolar molecule due to presence of electronegative oxygen atom as well as angular structure of water molecule.
So water attracts polar molecules through dipole-dipole attraction force and hence polar molecules get dissolved into water.
Water should have low temperature during dissolution of gas into water. Because, at high temperature, intermolecular force between gas and water molecule tend to break apart.
At high pressure, gas molecules are forced to enter into bulk liquid. Thus dissolution of gas into liquid becomes favorable.
Ответ:
Explanation:
It is known that water is a polar molecule due to the difference in electronegativity of hydrogen and oxygen atom there is a positive partial charge on hydrogen and a negative partial charge on the oxygen atom.
So, being a polar molecule water readily dissolve substance which are also polar in nature.
Hence, for a gas to be soluble in water it is required that the gas should contain polar molecules.
Also, water should be at low temperature because otherwise gas will evaporate from the solution due more gain in kinetic energy.
As, Henry's law states that the amount of gas dissolved or molar solubility of gas is directly proportional to the partial pressure of the liquid.
To calculate the molar solubility, we use the equation given by Henry's law, as follows.
where, = Henry's constant =
= Henry's constant = 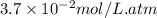
Therefore, pressure of the gas should be high at the surface of water.
Thus, we can conclude that for a gas to be soluble in water, the gas should contain polar molecules. The water should be at a low temperature, and the gas should be at high pressure above the surface of the water.
Ответ:
gas relaxation
Explanation: