bigmouth804
16.11.2019 •
Chemistry
For the decomposition of calcium carbonate, consider the following thermodynamic data (due to variations in thermodynamic values for different sources, be sure to use the given values in calculating your answer.): δh∘rxn 178.5kj/mol δs∘rxn 161.0j/(mol⋅k) calculate the temperature in kelvins above which this reaction is spontaneous.
Solved
Show answers
More tips
- A Animals and plants Уход за джунгариками: полезные советы и рекомендации...
- L Legal consultation What Documents Are Required for a Russian Passport?...
- S Sport How to Choose Tennis Rackets?...
- H Health and Medicine AKDS Vaccination: Ensure Your Child s Safety...
- H Health and Medicine Naskol ko Opasen Ukus Kleshcha i Kak Ego Raspoznat...
- C Computers and Internet How to Delete Your Account on Odnoklassniki...
- H Health and Medicine What to Do When Your Jaw Locks Up?...
- G Goods and services What Are the Most Popular Services?...
- P Philosophy How did the concept of module arise in computer science?...
Answers on questions: Chemistry
- E English The director and producer of the filmgiving an interview answer in subject...
- H History PLEASE HELP!!! What is the name of the movement that culminated in the passing of the 19th amendment Women s suffering Women s suffrage Women s protest Women s right to vote...
- M Mathematics Find the slope of the line...
- E Engineering A timing light checks the ignition timing in relation to the position....

Ответ:
Answer : The temperature in kelvins is,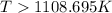
Explanation : Given,
Gibbs–Helmholtz equation is :
As per question the reaction is spontaneous that means the value of is negative or we can say that the value of
is negative or we can say that the value of  is less than zero.
is less than zero.
The above expression will be:
Now put all the given values in this expression, we get :
Therefore, the temperature in kelvins is,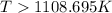
Ответ:
When we use this method for the reaction of C to CO2, the C in carbon dioxide has an oxidation number of 4+ while the two oxygens have an oxidation number of 2- . Clearly, the C has "lost electrons" and has become oxidized by interacting with the oxidizing agent O2.
Explanation: