For the following reaction, 4.07 grams of aluminum oxide are mixed with excess sulfuric acid. the reaction yields 10.4 grams of aluminum sulfate. aluminum oxide (s) + sulfuric acid (aq) aluminum sulfate (aq) + water (l) what is the theoretical yield of aluminum sulfate? grams what is the percent yield for this reaction? %
Solved
Show answers
More tips
- S Society and Politics What If There s War Tomorrow, What If We Go to War?...
- S Sport How to Do a Jumping Split...
- A Animals and plants What Do Terriers Eat?...
- F Food and Cooking Discover the Benefits and Properties of Dates...
- C Computers and Internet Dynamically Assigned IP Address: What Is It and How Does It Work?...
- S Style and Beauty How to Get Rid of Acne: Scientifically Proven Methods...
- H Health and Medicine Simple Ways to Lower Cholesterol in the Blood: Tips and Tricks...
- O Other How to Choose the Best Answer to Your Question on The Grand Question ?...
- L Leisure and Entertainment History of International Women s Day: When Did the Celebration of March 8th Begin?...
- S Style and Beauty Intimate Haircut: The Reasons, Popularity, and Risks...
Answers on questions: Chemistry
- C Chemistry A solution contains a mixture of pentane and hexane at room temperature. The solution has a vapor pressure of 256 {\rm torr}. Pure pentane and hexane have vapor pressures...
- C Chemistry Ireally don t know how to do stoichiomisrty...
- C Chemistry Compare the composition of sucrose purified from the sugar cane with the composition of sucrose purified from sugar beets....
- C Chemistry What is the formula for quick lime...
- C Chemistry You are caring for the freshwater aquarium of a friend while they are on vacation. You are told to check the pH of the tank and make sure that it stays between 6.5 and...
- C Chemistry Dimethylglyoxime, DMG, is an organic compound used to test for aqueous nickel(II) ions. A solution prepared by dissolving 65.0 g of DMG in 375 g of ethanol boils at 80.3°C....
- H History What was the area in between the trenches called during world war 1? a.) the great soldier zoneb.) the dead zonec.) no man s landd.) no tolerance land...
- M Mathematics What is the next number in the series? 76 69 62 55 48 ?...
- H History Which of the following principles is shared amongst islam judaism and christianity...
- B Biology Carotenoids are often found in foods that are considered to have antioxidant properties in human nutrition. what related function do they have in plants? a. they serve...

Ответ:
Theoretical yield = 13.7 g
% yield =76 %
Explanation:
For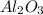
Mass of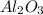 = 4.07 g
= 4.07 g
Molar mass of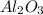 = 101.96 g/mol
= 101.96 g/mol
The formula for the calculation of moles is shown below:
Thus,
According to the reaction:
1 mole of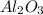 on reaction produces 1 mole of
on reaction produces 1 mole of 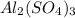
So,
0.0399 mole of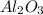 on reaction produces 0.0399 mole of
on reaction produces 0.0399 mole of 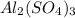
Moles of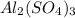 obtained = 0.0399 mole
obtained = 0.0399 mole
Molar mass of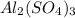 = 342.2 g/mol
= 342.2 g/mol
The formula for the calculation of moles is shown below:
Thus,
Theoretical yield = 13.7 g
The expression for the calculation of the percentage yield for a chemical reaction is shown below as:-
Given , Values from the question:-
Theoretical yield = 13.7 g
Experimental yield = 10.4 g
Applying the values in the above expression as:-
% yield =76 %
Ответ: