iancuteodora30
18.03.2021 •
Chemistry
For the reaction H 2 (g)+1/2 O 2 (g) H 2 O(l) Delta*G = - 237kJ / m * o * l AH = -286 kJ/ mol. Which statement is true? None of the other statements are true . The reaction is spontaneous . The entropy change is positive . More work can be done than just the thermal energy liberated by the reaction . Three of the other statements are true .

Solved
Show answers
More tips
- H Health and Medicine What was the Invention of Viagra?...
- P Philosophy Why Did God Create Man and Place Him in Obscurity?...
- S Science and Technology How to Make a Homemade Smoker: The Ultimate Guide...
- S Society and Politics 10 Tips for Boosting Your Self-Esteem...
- C Computers and Internet How to Create a Folder on Your iPhone?...
- G Goods and services How to sew a ribbon: Tips for beginners...
- F Food and Cooking How to Make Mayonnaise at Home? Secrets of Homemade Mayonnaise...
- C Computers and Internet Which Phone is Best for Internet Surfing?...
- F Food and Cooking Everything You Need to Know About Pasta...
- C Computers and Internet How to Choose a Monitor?...
Answers on questions: Chemistry
- C Chemistry Ineed asap! curious carl s chemistry teacher asked him to make a sugar solution. carl dissolved 50.0 grams of sucrose (c12h22o11, molar mass 342.3 g/mol) in 1.00 l of water....
- C Chemistry How many atoms are in a sample of 72.8 grams calcium ?...
- C Chemistry The heat of fusion of water is 80 cal/ g. how much energy is needed to melt 0.05 kg of ice?...
- C Chemistry You have 36.8 g of o2 gas in a container with twice the volume as one with co2 gas. the pressure and temperature of both containers are the same. calculate the mass of carbon...
- C Chemistry Bohr believed that electrons travelled in orbits about the atomic nucleus. a. circular b. elliptical c. irregular d. oval-shaped...
- C Chemistry ionic compounds in nature tend to cool slowly. this causes things like rubies sapphires emerald to form. Why?...
- C Chemistry Complete and balance the equation. Show your work on paper and submit a screenshot to Canvas. N_20_5 A. Not possible B. 2, 2,5 C. 2,5,2 D. 2,2,4...
- E English Drag each tile to the correct box. How should the sentences be organized to best summarize the passage...
- A Advanced Placement (AP) Strawberries are sold in 1-pound, 2 pound, and 5-pound boxes. Stacy wants to buy exactly 10 pounds of strawberries. What s are two ways that Stacy could buy exactly 10 pounds...
- B Business Lynx Inc. sells apparel merchandise to Dyson Inc. at a gross sales price of $980, credit terms 2/10, n/30 on July 10, 2020. Dyson pays for the merchandise in full on July 19,...

Ответ:
Answer is in the photo. I couldn't attach it here, but uploaded it to a file hosting. link below! Good Luck!
cutt.ly/4zZs6GM
Ответ:
The rocket reached its maximum height at 2 seconds.
The maximum height of the rocket is 36 yards.
Step-by-step explanation:
Quadratic equation:
In the format
The maximum height happens at the instant of time:
The maximu height is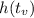
In this question:
So
When did the rocket reach its maximum height?
The rocket reached its maximum height at 2 seconds.
What was the maximum height of the rocket?
H(2).
The maximum height of the rocket is 36 yards.