Student3220
14.04.2020 •
Chemistry
Given the partial equation: H2O2 + ClO2 → O2 + ClO2−, balance the reaction in basic solution using the half-reaction method and fill in the coefficients. The missing blanks represent H2O, H+, or OH-, as required to balance the reaction. Enter the coefficients as integers, using the lowest whole numbers. If the coefficient for something is "1", make sure to type that in and not leave it blank. Enter only the coefficients. H2O2 + ClO2 + → O2 + ClO2− +
Solved
Show answers
More tips
- F Food and Cooking 10 Reasons Why You Should Avoid Giving Re-Gifts: An Informative Guide...
- S Sport How to wrap boxing hand wraps? Everything you need to know!...
- A Animals and plants 5 Tips for Taking Care of Yews to Keep Them Green and Beautiful...
- H Health and Medicine How to Calm Your Nerves? Expert Tips That Actually Work...
- O Other What is a Disk Emulsifier and How Does it Work?...
- S Sport How to Pump Your Chest Muscle? Secrets of Training...
- C Computers and Internet How to Get Rid of 3pic Infector: Everything You Need to Know...
- S Style and Beauty How to Grow Hair Faster: Real Methods and Advice...
- C Computers and Internet How to Top Up Your Skype Account Without Losing Money?...
- C Computers and Internet How to Get Rid of Spam in ICQ?...
Answers on questions: Chemistry
- B Business If you earned $10-an-hour in 2005 when the cpi was 100, and you earn $11-an-hour today when the cpi is 120, then your real wage rate has since 2005....
- H History Where did the highest number of Japanese American live?...
- B Biology All the subatomic particles participate in chemical reactions. true or false...

Ответ:
Answer : The balanced chemical equation in basic medium will be,
Explanation :
Redox reaction or Oxidation-reduction reaction : It is defined as the reaction in which the oxidation and reduction reaction takes place simultaneously.
Oxidation reaction : It is defined as the reaction in which a substance looses its electrons. In this, oxidation state of an element increases. Or we can say that in oxidation, the loss of electrons takes place.
Reduction reaction : It is defined as the reaction in which a substance gains electrons. In this, oxidation state of an element decreases. Or we can say that in reduction, the gain of electrons takes place.
The given chemical reaction is,
The oxidation-reduction half reaction will be :
Oxidation :
Reduction :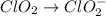
Now balance the hydrogen atom.
Oxidation :
Reduction :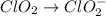
To neutralizing the hydrogen ion by adding hydroxide ion on both side.
Oxidation :
Reduction :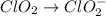
Hydrogen ion hydroxide ion combine to form water.
Oxidation :
Reduction :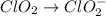
Now balance the charge.
Oxidation :
Reduction :
The charges are not balance on both side of the reaction. We are multiplying reduction reaction by 2 and then added both equation, we get the balanced redox reaction.
Oxidation :
Reduction :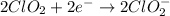
The balanced chemical equation in basic medium will be,
Ответ:
Mood-the overall atmosphere or feeling that a literary work creates for the reader
Tone-the author’s attitude toward the subject
Voice_the way authors, narrators, or speakers express their personality in a text